AMINES
AMINES
Amines are derivatives of ammonia (NH3) in which one or more hydrogen atoms have been replaced by alkyl groups.
CLASSIFICATION
Amines are classified as primary (1º), secondary (2º) or tertiary (3º) according to the number of alkyl groups attached to the nitrogen atom
The carbon atom of alkyl groups to which nitrogen is attached may be 1º, 2º or 3º but amine is always 1º, 2º or 3º if it has the grouping – eg.
eg.
STRUCTURE
The nitrogen atom in amine is sp3 hybridised. The three hybrid atomic orbitals are involved in bond formation and one hybrid atomic orbital contains the unshared pair of electrons, giving the pyramidal structure to amine.
The bond angles are approximately tetrahedral
NOMENCLATURE
ISOMERISM
Amines exhibit following types of Isomerism
Chain Isomerism
Functional Isomerism
Position Isomerism
Metamerism
METHODS OF PREPARATION
These methods yielding mixture of amines
HOFMANN’S METHOD (Ammonolysis of alkyl halide)
Using aqueous or alcoholic NH3
Using aqueous or alcoholic NH3
When NH3 is in excess, 1º amine is the main product
When RX is in excess, 3º amine is the main product
SABATIER METHOD (Ammonolysis of alcohols)
When NH3 is in excess, 1º amine is main product.
SEPARATION OF AMINE MIXTURE
HINSBERG’S METHOD
Reagent used is Benzenesulfonyl chloride (C6H5SO2Cl) which has been replaced by
p-toluenesulfonyl chloride, CH3C6H4.SO2Cl
Reagent used is Benzenesulfonyl chloride (C6H5SO2Cl) which has been replaced by
p-toluenesulfonyl chloride, CH3C6H4.SO2Cl
HOFMANN’S METHOD
Reagent used is Diethyl oxalate
METHODS GIVING PRIMARY AMINES ONLY
- Reduction of nitro compounds by Sn/HCl, H2/Ni, Zn/HCl or LiAlH4
- Reduction of cyanides (Mendius reaction)
- Reduction of oximes : or LiAlH4 are reducing agents
- Reduction of amide
- Hoffmann's bromamide reaction: R can be alkyl or aryl (Conversion of an amide to 1º amine)
- Gabriel’s phthalimide reaction
This method is not suitable for 1ºarylamine due to less nucleophilic character.
- From 𝛂-amino acids
- Reductive amination of Aldehydes and Ketones
- From Chloramine and Grignard’s Reagent
- Reduction of azide
- Ritter reaction : for amines containing tertiary alkyl groups
- By Lossen rearrangement : From Hydroxamic acid
- Schmidt reaction
- Curtius rearrangement
- By Leuckart reaction
PREPARATION OF SECONDARY AMINES
- From p-amines
- From calcium cyanamide
- Secondary amine containing phenyl group
- From 1º-amine by the action of diazomethane
- From aniline. It is one of the best methods
- Reduction of isocyanides
(Mendius Reaction)
- Reduction of N-Substituted amides
PREPARATION OF TERTIARY AMINES
- By heating ethanolic solution of ammonia with excess of alkyl halide
- Reduction of N, N-disubstituted amide
- Decomposition of alkyl tetraammonium hydroxides
GENERAL PROPERTIES
Amines are soluble in water due to H-bonding. Lower members have fishy ammoniacal odour and are combustible gases. From C3 to C11 volatile liquids and from C12 are solids. The b.p. increases with increase in molecular weight.
Basic character : Amines are stronger bases than ammonia
The stability of ions by H–bonding in water follows the order
The order of basicity ought to be 1° > 2° > 3° which is practically not.
In fact it depends upon this factor
- Inductive effect
- Steric hindrance
Basic character follows the order
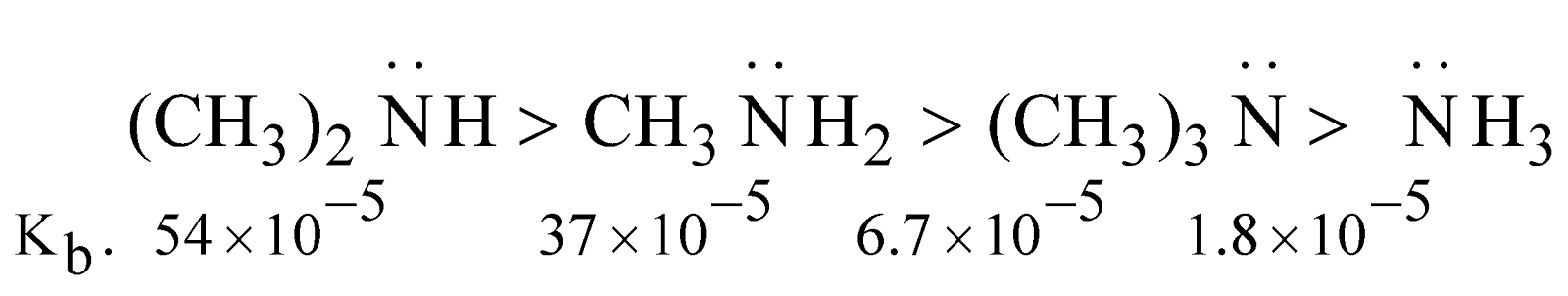
Ammonia and amines oscillate between two structures as follows
Although the electron density on nitrogen is maximum (due to + I effect of alkyl groups) in case of tertiary amine but approach and bonding by proton is relatively difficult due to steric hindrance. The steric hindrance increases with the bulk of group and basic character decreases
Alkyl group Relative strength
Note : The order of basicity of amines in the gaseous phase is
tertiary amine > secondary amine > primary amine > ammonia
Aromatic amines are less basic than aliphatic amine. The electron withdrawing groups (-NO2, COOH, –CN, –SO3H) decrease the basic character and electron donating groups (–CH3; –OH) increase the basic character of aniline
Due to Resonance the lone pair of electrons is delocalised within benzene nucleus and not available for protonation and basic character is suppressed.
Amides are much less basic than amines due to Resonance.
CHEMICAL PROPERTIES
AROMATIC AMINES
GENERAL METHODS OF PREPARATION
- Reduction of nitro compounds
- From aryl halides by action of ammonia
- Reduction of nitroso compounds
- Hoffmann’s bromamide reaction
CHEMICAL REACTIONS
BENZENE DIAZONIUM CHLORIDE : C6H5 N= NCl
PREPARATION
PROPERTIES
Colourless crystalline solid soluble in water. It has tendency to explode when dry
Replacement of - N2Cl group is unimolecular nucleophilic in nature
Replacement of - N2Cl group is unimolecular nucleophilic in nature
NITRO COMPOUNDS
Nitro compounds are characterised by the presence of nitro group (–NO2)
STRUCTURE OF NO2 GROUP
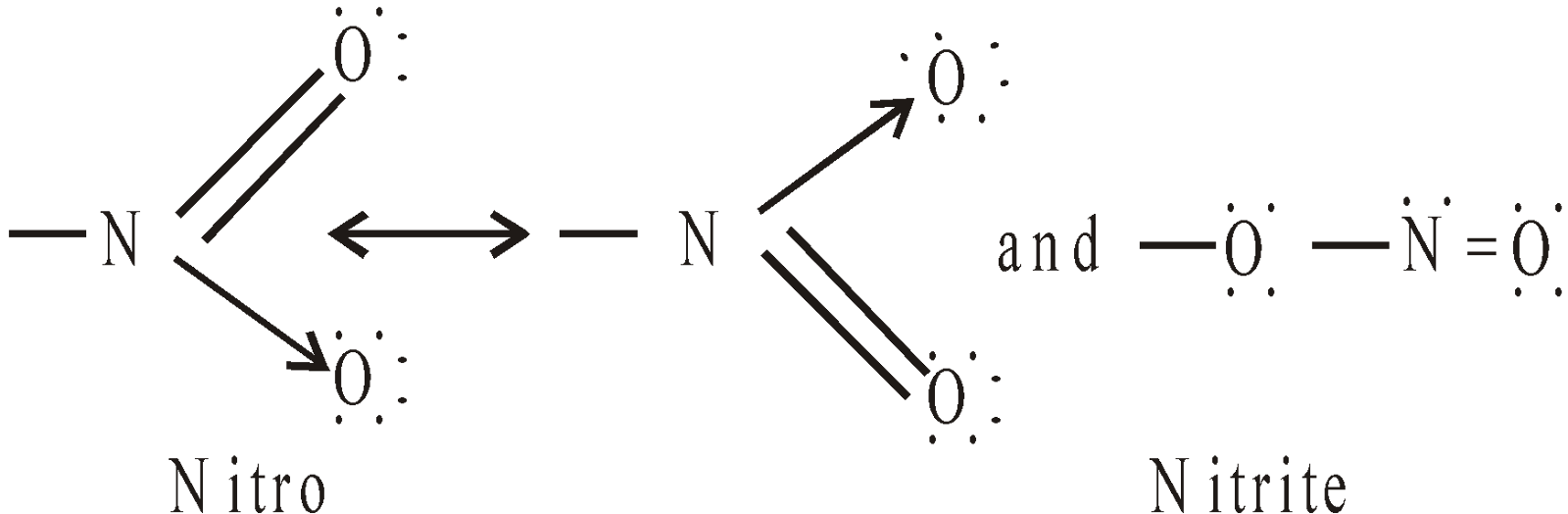
Hence – NO2 group is ambident group.
CLASSIFICATION
They may be primary, secondary and tertiary
NOMENCLATURE
They are named as nitroalkanes (aliphatic) and nitroarenes (aromatic)
PREPARATION OF ALIPHATIC NITRO COMPOUNDS
- Direct nitration of paraffins
Ease of replacement of hydrogen atom 3º > 2º > 1º
- From alkyl halides with AgNO2 or KNO2
- Hydrolysis of 𝜶-nitro alkenes
PREPARATION OF AROMATIC NITRO COMPOUNDS
Nitration is electrophilic substitution reaction. The electrophile is nitronium ion
- Mixture of conc. nitric acid + acetic anhydride gives better yield (Pictet and Kholinsky’s method)
- N2O4 + AlCl3 (Schaar’s Schmidt method)
Nitro benzene has high dipole moment 4.0D, bpt. 209ºC, it has odour of bitter almonds and also known as oil of mirbane.
CHEMICAL PROPERTIES OF NITROALKANES
CHEMICAL PROPERTIES OF ALKYL NITRITES
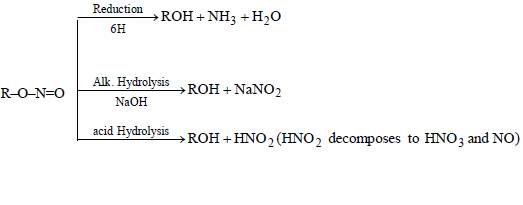 CHEMICAL PROPERTIES OF NITRO BENZENE
CHEMICAL PROPERTIES OF NITRO BENZENE
REDUCTION OF NITROBENZENE UNDER DIFFERENT CONDITIONS
ELECTROPHILIC SUBSTITUTION REACTIONS
METHYL ORANGE
It is used as an internal indicator. It gives yellow colour in alkaline medium and red colour in acid medium. Its pH range is 3.2 – 4.5.
ALKYL CYANIDES, NITRILES RC ☰ N
PREPARATION
PROPERTIES
Alkyl Cyanides are neutral with pleasant smell, moderately soluble in water
ALKYL ISOCYANATES: ISONITRILES OR CARBYLAMINE
PREPARATION
- Carbylamine reaction
PROPERTIES
They are colourless, extremely poisonous, unpleasant liquids. Insoluble in water. They have low bpt. than cyanides
USES
- For synthesis of 1º amines and acids
- Acrylonitriles (CH2 = CHCN) for manufacturing of polymers like orlon, acrilan fibres and rubbers etc.










