CHEMISTRY IN EVERYDAY LIFE
DYES
DYE : A synthetic or natural colouring matter to colour fabrics, plastics etc. The colour must be fast to the action of water, acid, alkalies, soap, light or friction. Every coloured substance is not a dye.
CHROMOPHORES : The groups with multiple bonds responsible for the colour of the compounds eg. NO2, NO, N = N, quinonoid structure etc.
AUXOCHROMES : The groups that intensify the colour of the dye and confer the salt forming property to it eg - OH, –NH2, –SO3H, –COOH, OR, –NHR, –NR2.
BATHOCHROMES : The groups that deepen the colour.
HYPSOCHROMES : The groups that lighten the colour.
Order of deepening the Colour
Yellow Orange
Orange  Red
Red  Purple
Purple Blue
Blue  Green
Green  Black.
Black.
MORDANT : A metal ion or a metal oxide that binds the fabric and the dye.
CLASSIFICATION ON THE BASIS OF CHEMICAL STRUCTURE
- NITRO DYES
Prepared by the action of nitric acid on phenols, Naphthols and amines.
- AZODYES (– N = N –)
Acidic azo dyes
Basic azo dyes
- TRIPHENYLMETHANE DYES
- PHTHALEIN DYES
- ANTHRAQUINONE DYES
- INDIGO
CLASSIFICATION ON THE BASIS OF APPLICATION
- DIRECT DYES : Generally acidic and basic dyes directly applied to a fabric.
Congo red dyes cotton full red. Another example is Martius yellow.
- VAT DYES : They are used in their reduced state (leuco compound) and after application to the fabric, are oxidised to the dye e.g. Indigo.
It binds to the cotton through H-bonding.
- MORDANT DYES : Do not dye fabric directly, they require a mordant. For acidic dyes, a metal hydroxide and for basis dyes tannin (tannic acid) is required. The metal salts have been referred to as lakes.
- INGRAIN DYES (AZOIC DYES) : These are insoluble azo dyes and directly produced on the fibre. (Cotton or cellulosic). e.g. Nitroaniline red.
- DISPERSE DYES : These are water insoluble dyes and can be dispersed in colloidal form in water by suitable reagents. The fine dye particles are absorbed into the crystal structure of the fabric. These are used with synthetic fabrics Nylon, Polyester etc. Examples.
CHEMOTHERAPY
CHEMOTHERAPY
Treatment of diseases by Chemicals having specific toxic effect upon the disease producing microorganisms without affecting the tissues of the host is called chemotherapy.
ANTISEPTICS
The chemicals which prevent or check the sepsis of wounds. Examples are : dettol (chloroxylenol + terpineol), Bithional (added to soaps). Salol, Acriflavine, Savlon, Gention violet, Mercurochrome, Salicylic acid, picric acid, resorcinol, phenol, iodoform, boric acid, iodine, methylene blue, potassium permanganate.
DISINFECTANTS
The chemical substances which completely destroy the microorganisms or stop their growth but are harmful to human tissues are called disinfectants. Examples are : 1.0% phenol, SO2 in very low concentrations and chlorine in 0.2 to 0.4 ppm. They are used to disinfect floors, clothes, utensils etc.
ANTIPYRETICS
They lower the body temperature i.e. fever reducing. Examples are : aspirin, paracetamol, phenacetin, analgin.
ANALGESICS
They are pain relieving. Examples are : Aspirin and analgin, both are antipyretic and analgesics. Novalgin is most widely used analgesic. Certain narcotics like Codeine, morphine, pethidine hydrochloride, methadone and heroin etc. are also used as analgesics.
TRANQUILIZERS
Also called psycho-therapeutic drugs, reduce anxiety, induce sleep, and cure mental diseases. Examples are : Barbituric acid and its derivatives, seconal, luminal, Methyldopa and Hydralazine, Equanil, Chlordiazepoxide.
SEDATIVES AND HYPNOTICS
They are central nervous system depressants reduce restlessness, emotional tension and induce sleep. Examples are : Phenobarbital, Glutethimide, Valium, Veronal, Serotonin etc.
ANTIANXIETY AGENTS
Examples are : Meprobamate and Diazepam, Iproniazid, Phenelzine (Nardic)
Tranquilizers, Sedatives & Hypnotics and Antianxiety agents are central nervous system stimulants.
ANAESTHETICS
The chemical substances which produce insensibility to the vital functions of all types of cell especially of nervous system temporarily are called anaesthetics. Examples are, General anaesthetics - Chloroform, Fluothane, Local anaesthetics - Cocaine, a-Eucaine, b-Eucaine.
NARCOTICS
The chemical substances which act as depressant and analgesic. Examples : Heroin, Opium and Pethidine.
ANTIBIOTICS
The chemical substances produced by microorganisms or by chemical synthesis that inhibit the growth of bacteria (bacteriostatic)or even destroy (bactericidal) them are called antibiotics. Examples : Penicillin is the first antibiotic discovered by Alexander Fleming. It is effective against pneumonia, bronchitis and sore throat etc.
when 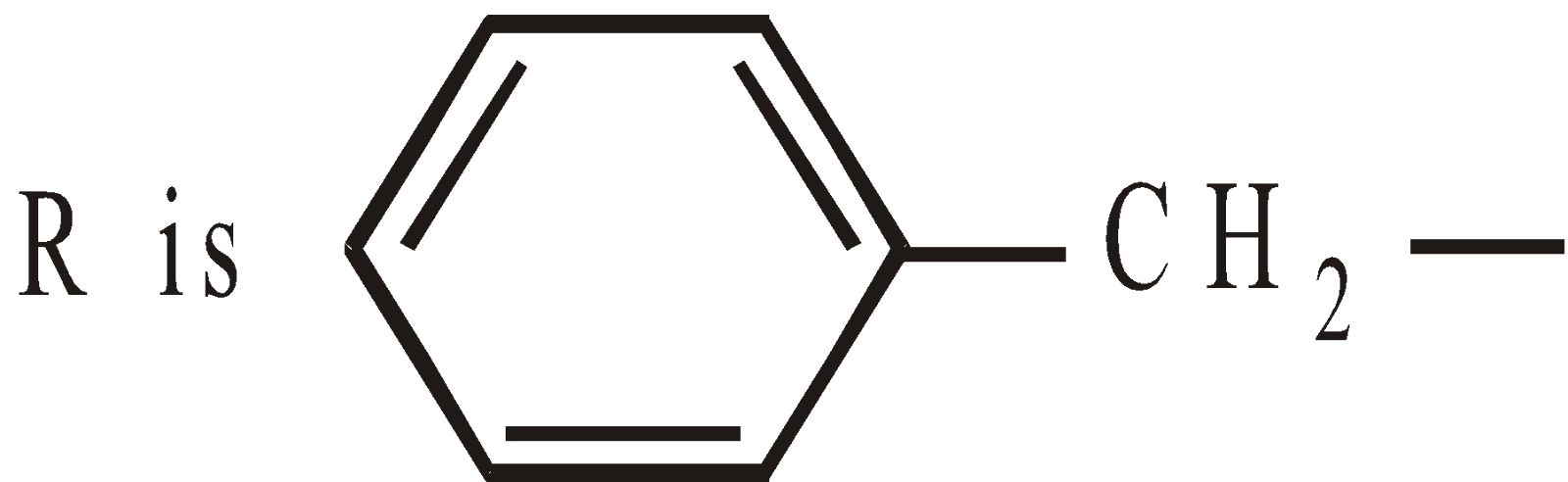 Penicillin G or Benzyl penicillin
Penicillin G or Benzyl penicillin
Spectrum : The complete range of micro organism that can be killed by a particular antibiotic is known as spectrum. These are of following types:
- Narrow Spectrum antibiotics : Streptomycin, Chloromycetin
- Broad Spectrum antibiotics : Chloramphenicol, Tetracycline
Streptomycin tuberculosis is used in treatment
Chloramphenicol is used in the treatment of typhoid, dysentery, whooping cough, certain primary infections and meningitis.
Ampicillin though works against large number of diseases, induces allergy. Hence allergy test is must before its administration.
Examples of the types of antibiotics are
- Bacteriocidal (Killing) – Aminoglycosides, Ofloxacin and Penicillin.
- Bacteriostatic (Inhibitory) – Tetracycline, Erythromycin and chloramphenicol
SULPHA DRUGS
Sulpha drugs possess powerful bacteriostatic property due to Sulfonamide group.
Sulphadiazine (Sulpha pyrimidine) : For pneumonia, rheumatic fever and bacillary dysentery
Sulphanilamide : For pneumonia, urinary and respiratory infections
Sulphathiazole (Cibazol) : For staphylococci infections, bubonic plague
Sulpha acetamide : For urinary tract infections
ANTIMALARIALS
These are the drugs which cure malaria. Examples are : Plasmoquine (Plasmochin), Atebrin (Mepacrine), Chloroquine.
ANTIMICROBIALS
Antimicrobials are the drugs which are used to cure diseases caused by microbes or microorganisms. An antimicrobial may control the microbials diseases as a bactericidal drug (kill the microorganisms in the body) or as a bacteriostatic drug (inhibit the growth of microorganisms) or by increasing resistance of the body to infections.
Examples are:
ANTACIDS
Antacids are the drugs which neutralize excess acid in the gastric Juices and give relief from acid indigestion. They remove the excess acid and raise the pH to appropriate level in stomach. There are mainly weak bases.
Examples– Mg(OH)2, KHCO3, Omeprazole, Lansoprazole, Histamine, Cimetidine and Ranitidine.
ANTIFERTILITY DRUG
The drugs which are used to control the pregnancy are known as Antifertility drugs or oral Contraceptives. These are essentially a mixture of estrogen and progesterone derivatives. Examples – Ormeloxifene, mifepristone, Norethindrone, Ethynylestradiol (nonvestrol).
ANTIHISTAMINES
Antihistamines are the drugs which diminish the main action of histamine (Chemical substance which cause allergic reactions in body) released in the body and thus prevent the allergic reactions. These are also anti-allergic drugs. Examples diphenhydramine (Banadry) hydrochlorid, Pheniramine maleate (Avil), Brompheniramine (Dimetapp), terfenadine (seldane)
ROCKET PROPELLANTS
ROCKET PROPELLANTS
Rocket propellant is a combination of a fuel and an oxidiser which when ignited generates a large volume of hot gases at a predetermined rate.
SOLID PROPELLANTS
- Composite propellants : Fuel is a synthetic rubber or resin such as polyurethane or polybutadiene with additives such as Al or Mg. Oxidiser is Ammonium Perchlorate, potassium perchlorate or Amm. nitrate.
- Double base propellant : Mixture of nitroglycerine and nitrocellulose.
In solid propellants ignition can not be controlled.
LIQUID PROPELLANTS
- Biliquid propellants : Fuel is Kerosene, Alcohol, Hydrazine, Monomethyl hydrazine (MMH) and unsymmetrical dimethyl hydrazine (UDMH), liquid hydrogen. Oxidiser is liquid oxygen, dinitrogen tetraoxide or nitric acid.
- Monopropellants : Nitromethane, methyl nitrate hydrazine, and hydrogen peroxide. They act as fuel as well as oxidiser.
ADVANTAGES OF LIQUID OVER SOLID PROPELLANTS
- They provide higher thrust than solid propellants
- Thrust can be controlled
HYBRID PROPELLANTS
Fuel is solid acrylic rubber and oxidiser is dinitrogen tetroxide.
REQUIREMENTS OF GOOD PROPELLANT
Production of large volume of gases per gm of fuel and no residue left.
SOAPS AND DETERGENTS
(Lo detergere = to wipe clean). Any cleansing agent including soap as well.
Syndets : Synthetic detergents.
SOAPS
Metallic salts of higher fatty acids. Common higher fatty acids are palmitic acid C15H31COOH, stearic acid C17H35COOH and oleic acid C17H33COOH.
Hard Soaps : Sodium salt of higher fatty acids prepared from cheap oils contain free alkali also.
Soft Soaps : Potassium-salts of higher fatty acid.
Transparent Soaps : They contain glycerol and prepared by dissolving toilet soaps in ethanol.
Medicated Soaps : Soaps containing antiseptics, germicides etc.
Marine Soaps : Prepared from coconut oil and gives lather with sea water.
CLEANING ACTION OF SOAPS
It may be due the following two factors
- Solubilisation of grease into the micelle
- Emulsification of grease
When soap is applied on to a fabric, the tails of the soap anions are pegged into the grease stains and polar head form a charged layer around it. By mutual repulsion the grease droplets are suspended in water (formation of emulsion) and are washed away with water.
DETERGENTS
Sodium salts of alkyl hydrogen sulphates or long chain alkyl benzene sulphonic acids.
Anionic Detergents
Cationic Detergents / Invert Detergents
Nonionic Detergents : Monoesters of polyhydroxy alcohols or polyethene.
SUPERIORITY OF DETERGENTS TO SOAPS
With hard water soaps form insoluble Ca and Mg carboxylates. Detergents form Ca and Mg soluble salts.
CLEANSING ACTION OF DETERGENTS
The cleansing action of detergents are same as that of soaps. For example. A detergents, Sodium lauryl sulphate, CH3(CH2)OSO3Na, Contains the polar group OSO3– alongwith the long hydrocarbon chains. It is an anemic detergent in which anions associate together to form an ionic micelle. Similarly, Cationic detergent also forms micelle.
CHEMICALS IN COSMETICS
COSMETICS
Cosmetics are the substances used to improve a person's appearance by beautifying and improving complexion of skin.
CREAMS
Creams are semi-solid preparations applied on the face to prevent the skin from drying and to provide necessary moisture, nutrients and vitamins to keep it healthy.
The creams are of the following types :
- Cold cream : These are water in oil type emulsions containing vegetable or mineral oil, fat, beeswax, lanolin (perfume), borax and emulsifier. They serve as moisturiser, lubricate the skin and protect it against wind.
- Vanishing cream : These contain stearic acid, alkali water and humectant (glycerine). These keep the skin cool and oily. Vanishing cream provides a thin film on the skin having higher melting point than skin temperature and it seems to disappear (vanish).
- Cleansing cream : These remove facial make up, surface grime (sooty dirt) and oil.
- Bleach cream : These bleach the skin to make it appear fair.
- Sunburn cream : These save the skin from sunburn in summer.
- Foundation cream : These provide a base for powder and make-up.
TALCUM POWDERS
Talc is a soft mineral having form of magnesium silicate 3MgO.4SiO2.H2O. In the powder form it is used on the skin to make it feel smooth, dry and irritation free which is known as talcum powder. Modern face powders and baby powder protect skin from heat, absorb perspiration and impart brightness to skin by covering blemishes and imperfections.
The important ingredients of the talcum powders are:
- Talc or talcum which is hydrated magnesium silicate 3MgO.4SiO2.H2O. Since talcum causes inflammation to skin by forming silicic acid, therefore aluminium hydrolicate is prefered.
- Adherents : Zn and Mg soaps e.g. Zinc stearate
- Absorbents : Chalk (Ca and Mg Carbonates) absorbs perspiration without any sign of absorption.
- To provide opacity : ZnO, TiO2 and colloidal clay and used. These mask the enlarged pores and minor blemishes and protect the skin from UV radiations.
- Specific ingredients : Cooling agents and antiseptics eg. boric acid, etc.
Baby powders generally contain Zinc stearate as adhesive and boric acid as antiseptic. The particle size of good talcum should be more than 70 micron.
DEODORANTS
The substances which remove or conceal unpleasant bodily smells are known as deodorants. They generally possess antibacterial properties and prevent bacterial decomposition of sweat by inhibiting, killing or removing the bacteria responsible for unpleasant body odours. Some deodorants mask the unpleasant body odours with more pleasant fragrances. Aluminium salts such as aluminium sulphate, aluminium chloride, aluminium hydroxychloride, aluminium alcoholate, aluminium chloro hydroxy acetate possess excellent antibacterial properties.
In addition to aluminium salts zinc oxides (ZnO, ZnO2) and zinc stearate are used since they are astringents (check bleeding) and antiseptics.
Phenolic antibacterials viz. p-chloro meta xylenol, dichlorometaxylenol and bithional are used in soaps, creams, baby powders, shaving lotions as body deodorants.
PERFUMES
A perfume may be defined as a mixture of pleasant smelling substances incorporated in a suitable solvent. It is composed of three ingredients given as follows :
- Vehicle or solvent - To keep the pleasant smelling substances together a solvent is required which is also known as vehicle. Water-ethanol mixture is the most common vehicle which is volatile, odourless and non irritant.
- Fixative : The substance which equalise the rate of evaporation of various odoriferous or odour producing components of the perfume. The fixatives may be
- Essential oil fixatives : Sandalwood (most common), orris patchouli, vetine etc.
- Animal fixatives : Musk, civet, ambergris castor etc.
- Resinous fixatives : Benzoin, tolu balsam, perubalsam, terpenoids etc.
- Synthetic fixatives : Glycerol diacetate, benzyl benzoate, ethyl phthalate etc.
- Odoriferous substances : These may be natural or synthetic.
NATURAL PERFUMES
They are obtained from plants and animals. eg. essential oils (terpenes) linalool, limonene, camphor, pinene, geraniol, citronellol eugenol, cineole, citral, bantalol. The perfumes derived from animal sources are muscone, civet, caster musk, ambergris, musk zibata etc.
SYNTHETIC OR SEMISYNTHETIC PERFUMES
- Esters : Esters of benzyl alcohol, cinnamic acid, salicylic acid. Common are benzyl propionate, isobutyrate, methyl salicylate (oil of wintergreen), benzyl cinnamate
- Alcohols : 2-hexenol, 3-hexenol, phenyl ethyl alcohol.
- Nitro compounds : musk xylene and musk ambrette.
- Aldehydes : Anisaldehyde (p-methoxybenzaldehyde) and cinnamaldehyde etc.
- Biphenyls : Diphenyl ether, biphenyl methane.
- Ketones : Exaltone, benzophenone.
CHEMICALS IN FOOD
FOOD ADDITIVES
The chemicals, synthetic or natural substances added to food preparations for different purposes as given below are known as food additives.
- Nutrients : To increase the nutritive value of the food eg. carbohydrates, proteins etc.
- Preservatives : To retard spoilage from bacterial action. eg. NaNO2 and NaNO3 (in meat), C6H5COONa (in tomato ketchup, fruit juices), Sodium metabisulphite (in pickles), Citric acid (fruit drinks), Sodium propionate (in bread and cheese) and SO2 (in wine and juices).
- Flavour agents : To enhance the flavour or to develop flavour eg. alkyl alkanoates (esters), monosodium glutamate (MSG), vanillin, cinnamaldehyde.
- Antioxidants : To exclude oxygen to retard or prevent spoilage eg. BHT (Butylated hydroxytoluene) and BHA (Butylated hydroxyanisole).
- Sweeteners : To add sweet taste eg. saccharin aspartame, sucralose.
- Colourants : To give colour eg. Amarnath, Kesar etc.
CERAMICS
They are inorganic, non metallic materials made of clay that is permanently hardened by heat at high temperature.
CLAY
Certain earths, which are highly plastic when wet and converted to hard mass when heated which is unaffected by water. Clay is essentially hydrated aluminium silicate (Al2O3.2SiO2.2H2O). Clay with a low content of iron is usually white, so it burns white and is called Kaolin or China Clay.
TYPES OF CERAMICS
broadly they are of three types:
- Clay products : porcelain, pottery, sanitary fittings, tiles, sewer pipes etc.
- Refractories : Refractory bricks used as furnace linings
- Glasses : Kitchenware
REFRACTORY
Any material that can withstand high temperature without softening or suffering from deformation in shape.
CLASSIFICATION OF REFRACTORIES
They have been classified into three main types
- Acid refractories : Which consists of acidic material like SiO2, Al2O3. They are attacked by basic materials.
- Basic refractories : Which consists of basic materials like CaO, MgO and attacked by acidic materials.
- Neutral refractories : They are made from weakly acid/base materials like Zirconia ZrO2, Carbon, Chromite (FeO, CrO2).
- Abrasive ceramics : The silicon and tungsten carbides are very hard and used as cutting and grinding tools.
- Superconducting ceramics : These consists of mixture of oxides of metals eg. Yttrium Oxide, barium oxide, copper oxide, etc. They are used to electrical power transmission, building powerful magnets, navigation transportation, nuclear fusion experiments.
INSECT ATTRACTANTS/REPELLENTS
SEX ATTRACTANTS / PHEROMONES
These are chemical substances secreted by insects used as signals (peculiar odour, colour etc.) by the other members of the same family. These are very powerful and required in very small amount (10–10–10–20g/cm3). Some common sex attractants are as follows :
- Bombykol
1-hydroxy-trans 10-cis-12-hexadecane-diene
It is secreted by the abdominal cells of the silkworm moth. - Eugenol
- Muscaline or (Z)-9-Tricosene
- 9-oxo-trans-2-decenoic acid
It is queen-bee sex attractant. - Dispariline : secreted by female gypsy moth.
INSECT REPELLENTS
These are chemical substances used to drive out the troublesome insects rather than killing them. The following are common mosquito repellent
Di-n-propyl-2. S-pyridinedicarboxylate is used or housefly repellent.
Pest control : By the use of sex attractants particular insects can be collected at one place and later killed by usual insecticides without killing the desired insects which are otherwise useful.










