COORDINATION COMPOUNDS
DOUBLE SALTS
The addition compounds which retain their identity in solid form only and not in solution are known as double salts eg carnallite.
In aqueous solution carnallite shows the properties of K+, Mg2+ and Cl– ions
COORDINATION COMPOUNDS
The addition compounds which retain their identity in solid as well as in solution. Such compounds contain a complex ion formed by the combination of a metal atom and other species having lewis base character eg.
Since the complex ion contains a number of coordinate bonds they are also known as coordination compounds.
SOME DEFINITIONS
COMPLEX
A central metal atom /ion surrounded by a set of ligands.
LIGAND
An ion or a molecule that can have an independent existence and can donate a pair of electrons. It can be negative ion, neutral molecule or positive cation (though rare in nature).
COORDINATION COMPOUND
A neutral complex or ionic compound in which at least one of the ions is a complex formed between a lewis acid (-acceptor) and lewis base eg.
Ni = lewis acid centre, NH3 = Lewis base
Complex ion is always written in square brackets
COORDINATION NUMBER
Number of ligand donor atoms (not number of ligands) in a coordination compound (or complex) or number of electron pairs arising from ligand donor atoms to which the metal is directly bonded. Coordination number range from 1 to 12 (> 12 for some f- block element.
TYPES OF LIGANDS
- UNIDENTATE - Which binds to a metal through a single point of attachment eg. Br etc.
- BIDENTATE - Which binds to a metal through two points eg.
- POLYDENTATE - Several donor atoms are present in one molecule
(Dentate- derived from teeth)
CHELATE COMPLEX
It is formed when a bi or polydentate ligand uses two or more donor atoms to bind to one metal atom (“chelate” derived from claw). Most common elements to act as donor atoms are N, P, O, S halides and C ( in organic metallic compounds)
BRIDGING LIGANDS
Such ligands can bind to more than one metal atom
HOMOLEPTIC LIGAND
Metal bound to only one type of donor group
HETEROLEPTIC LIGAND
Metal bound to more than one type donor group
COORDINATION SPHERE
The combination of central metal atom and ligands written in “square brackets” is called the coordination sphere.
IONISATION SPHERE
The portion present outside the square bracket is called ionisation sphere.
Species present in the coordination sphere are non ionisable and species present in the ionisation sphere are ionisable.
OXIDATION NUMBER
Charge carried by the central metal atom
EFFECTIVE ATOMIC NUMBER (EAN)
It is equal to the number of monodentate ligands, twice the number of bidentate ligands and so on. It can be obtained from the following simple expression:-
EAN = Z – O.N + 2 (CN)
where Z = Atomic number of central metal atom
O.N. = Oxidation number of central metal atom
CN = Coordination number of central metal atom
WERNER’S THEORY
According to Werner’s theory metals have two types of linkages (valencies)
PRIMARY LINKAGES
Which are satisfied by the negative ions, ionisable and their number is equal to the O . N of central metal atom. They are always represented by dotted lines.
SECONDARY LINKAGES
Which are satisfied by the negative, neutral or a positive species (ligands) and their number is equal to the coordination number of the central metal atom. These are non ionisable and represented by complete lines. Every metal atom has a tendency to satisfy both the valencies. On the basis of Werner’s theory the structure of CoCl3.6NH3 can be represented as follows:
NOMENCLATURE OF COORDINATION COMPOUNDS
Nomenclature of coordination compounds follows different rules which are as follows.
- Name the cation, then anion
- Non ionic compounds are given one-word name
- Name ligands
- Ligands are named first and central atom last
- Ligands are named in alphabetical order
- Neutral ligands are named the same as the molecule (except aqua and ammine)
- Anionic ligands are named by adding - O to the stem of the name (chloride becomes chloro)
- The ligand name is preceded by a numerical prefix to indicate how many are present - di, tri, tetra, penta, hexa
- In a neutral or cationic complex, the name of the central metal atom is followed by its oxidation number in Roman numerals in parentheses
- In anionic complex, the suffix- ate is added to the name of central metal, followed by its oxidation number in Roman numerals in parentheses
- In case of bridging ligand the word m (mu) is written before the name of ligand
Formula and names of some ligands
H2O aqua
| ||
CO carbonyl
| ||
NH3 ammine
| ||
NO nitrosyl
| ||
C6H5 Phenyl
| ||
C5H5N Pyridine
| ||
PH3 Phosphine
| ||
P(C6H5)3 Triphenylphosphine
| ||
H2N. CSNH2 Thiourea
| ||
H2N. CH2. CH2. NH2 ethylenediamine
| ||
I–iodo
|
ligands carrying positive charge have ending of –ium
(NH2NH3)+ hydrazinium
ISOMERISM IN COORDINATION COMPOUNDS
ISOMERS
two or more forms of a compound having the same composition
- Structural Isomers - (have different bonding) . They are of the following types
- Ionization Isomers - Exchange ion between ligand and anion eg.
- Hydration Isomers - Exchange water as ligand and hydrate
- Linkage Isomers - ligands that can bond at more than one atomic site
(ambidentate) 
COORDINATION ISOMERISM
Occurs when cationic and anionic complexes of different metal ions are present in a salt. Interchange of ligand between the complexes give isomers eg
LIGAND ISOMERISM
Occurs when more than one isomer of the ligand is possible eg 1, 2 diaminopropane and 1,3-diaminopropane.
POLYMERISATION ISOMERISM
The isomers have the same empirical formula but different molecular weights eg.
 and
and 
The molecular weight of the second is twice as that of the first.
VALENCE ISOMERISM
In this the same coordinating group is held by different types of valence bonds. The valence state of the central metal atom then differ in the two isomers. eg.
In the first compound the NO group is a negative group and oxidation state of Co is +3. In the second compound the NO group is neutral and oxidation state of Co is +2.
COORDINATION POSITION ISOMERISM
In this coordinating groups occupy different positions and the isomerism occurs generally in bridged complex. eg.
STEREO ISOMERISM
It is due to different spatial arrangement of atoms and groups in a molecule. It is of two types.
GEOMETRICAL ISOMERISM
It is due to different geometrical arrangements of ligands around central metal atom and is shown by
- Square planar complexes of the type : MA2X2 ; MABX2 ; MABXY
Type MA2X2–
Positions 1,2 and 1,4 are cis while 1,3 and 2,4 are trans.
Type MABX2
[ Co (NH3)2 Cl Br ]
Type MABXY
Example is 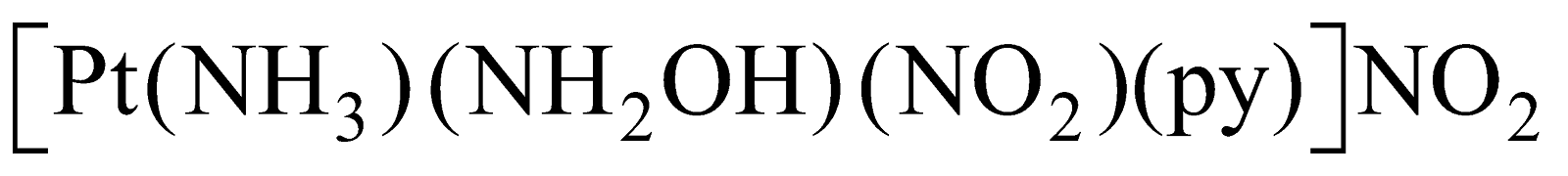 . Its three isomers are possible.
. Its three isomers are possible.
Type M(AA1)2 - Where AA1 is unsymmetrical bidentate eg [Pt(Gly)2],
here gly = 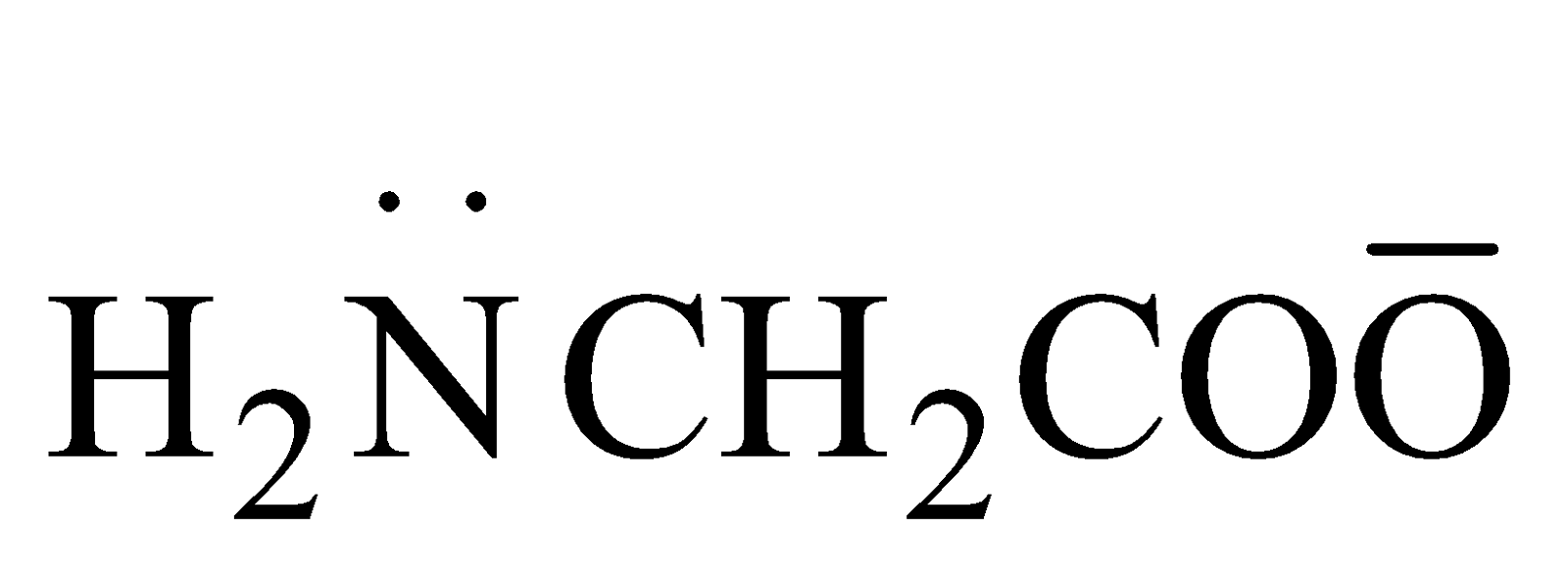
- Octahedral of the type : MA4XY, MA4X2 MA3X3 MA2X2Y2. M(AA)2X2 and M(ABCDEF)
In the last type 15 geometrical isomers are possible.
AA= symmetrical bidentate eg.
Type MA4X2
Note : The positions 1,6 and 2,4 and 3,5 are trans.
Type MA3X3
The geometrical isomerism is not possible in square planar MA4, MAB3 and tetrahedral MA4, MA2B2 and MABCD and Octahedral MA6, MA5B.
Facial (fac) meridional (mer) isomerism
The octahedral coordination compounds of the type MA3B3 eg. Co(NO2)3(NH3)3 exhibit this type of geometrical isomerism of each trio of donor atoms occupy adjacent positions at the corners of an octahedral face the isomer is known as facial (fac) isomerism. When the positions are around the meridian of the octahedron we have meridional (mer) isomer.
The octahedral coordination compounds of the type MA3B3 eg. Co(NO2)3(NH3)3 exhibit this type of geometrical isomerism of each trio of donor atoms occupy adjacent positions at the corners of an octahedral face the isomer is known as facial (fac) isomerism. When the positions are around the meridian of the octahedron we have meridional (mer) isomer.
OPTICAL ISOMERISM
Non superimposable mirror images are called optical isomers and may be described as “chiral’. They are also called enantiomers and they rotate plane polarised light in opposite directions.
Optical isomerism - It is given by octahedral complexes of the type
M(AA')3 (Cis or trans) M(AA)3 ; M(AA)2 B2 (Cis not trans) ; M(AA)2 BC (Cis form) ; M(AA)B2C2 , MA2B2C2 (Cisform), MA2B2CD, MA2BCDE, MABCDEF.
All exist in three forms two optically active and one optically inactive. Examples are
Tetrahedral Complexes
Tetrahedral complexes of the type (M(AA1)2 show optical activity. Examples are Bis(benzoylacetone) Be (ii) and Bis (glycinato) Ni (ii)
Tetrahedral complexes of the type (M(AA1)2 show optical activity. Examples are Bis(benzoylacetone) Be (ii) and Bis (glycinato) Ni (ii)
Tetrahedral complexes can have optical isomers if all four ligands are different eg. MABCD
VALENCE BOND THEORY
Features of this theory are:-
- Uses hybrid orbitals to hold the donated electron pairs for the formation of the coordinate bonds.
- Can explain the structure and magnetic properties eg consider the ions
First Example - 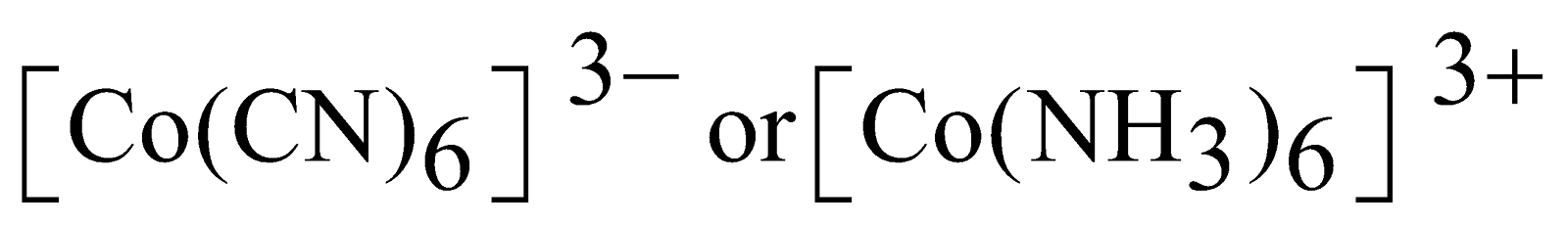
Outer electronic configuration of Co At . No. 27
Outer electronic configuration of Co3+
The coordination number is six. We need six empty atomic orbitals to accommodate electrons donated by CN or NH3.
d2sp3 hybridisation is octahedral. As shown above there is no unpaired electron hence the complex ions are diamagnetic in nature. It is inner complex since d atomic orbitals come from inside and low spin complex
Second example - Structure of 
Outer electronic configuration of Fe(At. No 26)
Outer electronic configuration of Fe2+
The coordination number is six. We need six empty valence atomic orbitals.
H2O is a weak ligand, pairing of electrons is not possible hence hybridisation sp3d2. Hence  is octahedral, paramagnetic in nature, outer complex and high spin complex.
is octahedral, paramagnetic in nature, outer complex and high spin complex.
Third example - Structure of 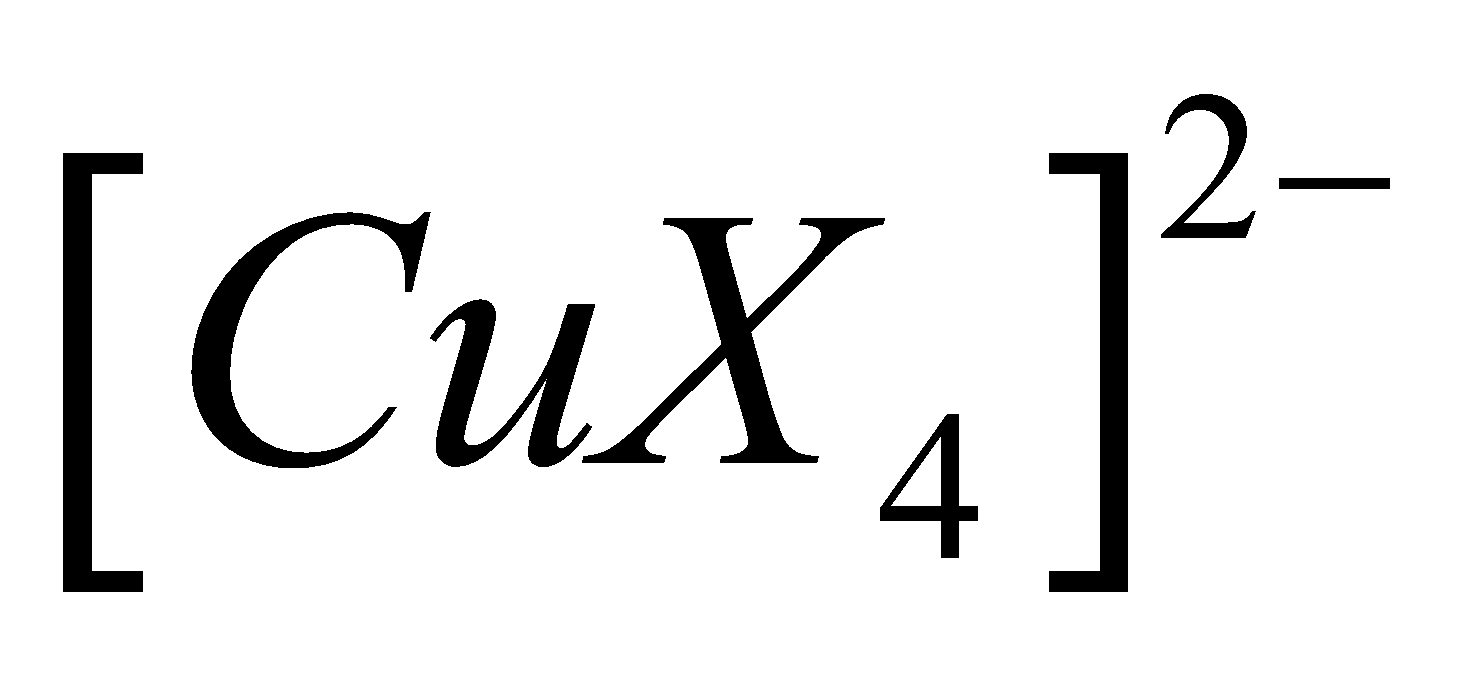
Cu2+ has electronic configuration
Since the coordination number is 4, we need four hybrid atomic orbitals. Hybridisation should be sp3 but X-ray analysis reveals the presence of four ligands in the same plane hence hybridisation should be square planar. For this Cu2+ should have the configuration.
Hence is 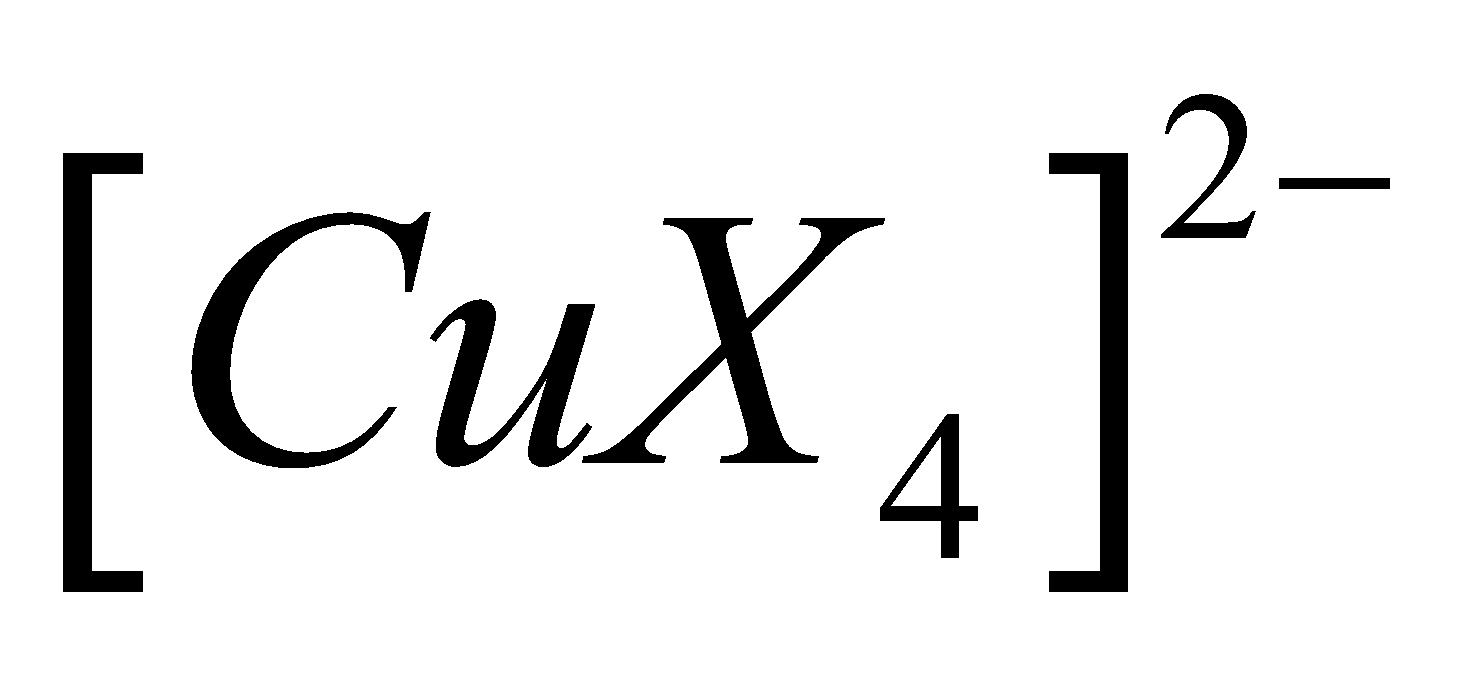 square planar, paramagnetic and inner complex.
square planar, paramagnetic and inner complex.
LIMITATIONS OF VALENCE BOND THEORY
- It does not explain the relative stability of complexes.
- It can not explain colour and spectra
- The relative stability of structural isomers.
CRYSTAL FIELD THEORY
Crystal field theory (CFT) was proposed by Bethe and Ven Vleck. It gives satisfactory explanation for the properties and bonding in coordination compounds. The main points of this theory are following :-
- The attraction between the central metal and ligands in the complexes is considered to be purely electrostatic Thus bonding in the complex may be ion-ion attraction or ion dipole attraction.
- Ligands are treated as point of negative charges
- There is no interaction between metal orbitals and ligand orbitals
- The d-orbitals present in metal have the same energy in the free state. This is called degenerate state of d-orbital. But, when a complex is formed the ligands destroy the degeneracy of these orbitals. This effect is Known as Crystal field splitting of d- orbitals.
It accounts for both the colour and the magnetic properties of complexes. It is based on d- orbital energy level splitting
Size of D depends on
- The nature of the ligand “Spectrochemical series” D decreases as shown below
- The oxidation state of the metal D is greater for M3+ than for M2+
- The row of the metal in the periodic table. For a given ligand and oxidation state of the metal,
increases going down in a group eg.
is greater in [Ru(NH3)6]3+ than in [Fe(NH3)6]3+
Effect of strong field ligands and weak field ligands
Strong field ligands forces the electrons of central metal for pairing and the complex formed is known as low spin complex. While weak field ligands do not forces the electron of central atom for pairing and the complex formed is known as high spin complex.
For example– In [Ni (CN)4], CN is a strong field ligand which forces electrons of Ni 2+ for pairing
Strong field ligands forces the electrons of central metal for pairing and the complex formed is known as low spin complex. While weak field ligands do not forces the electron of central atom for pairing and the complex formed is known as high spin complex.
For example– In [Ni (CN)4], CN is a strong field ligand which forces electrons of Ni 2+ for pairing
Ni 2+; [Ar] 3d8 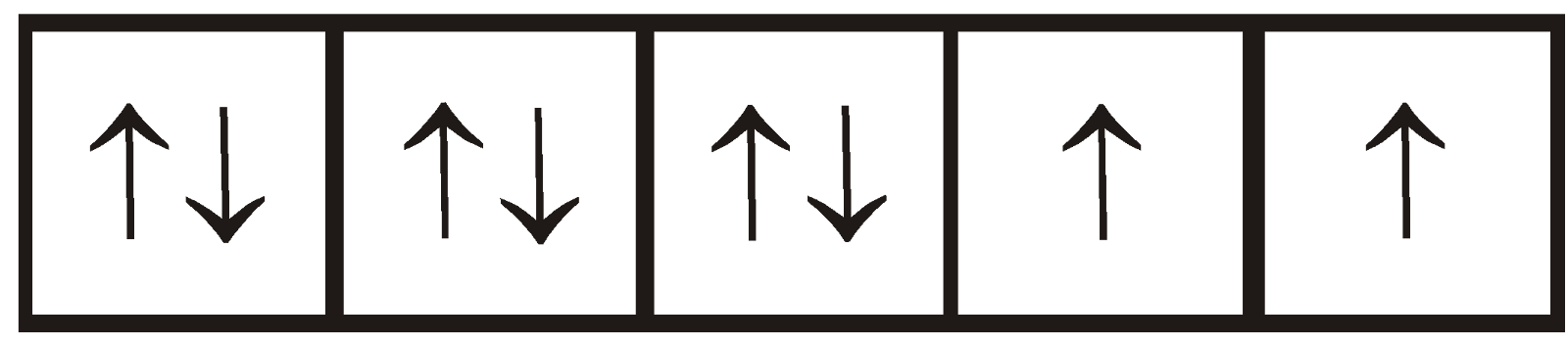
[Ni(CN)4]2– : [Ar]
In [NiCl4]2– , Cl– is a weak field ligand which do not forces electrons of Ni2+ for pairing
[ Ni Cl4]2– : [Ar]
Hybridisation : sp3 (tetrahedral)
COLOURS OF METAL COMPLEXES
It is due to electronic transitions between t2g and eg energy levels. The energy of an electron is increased by absorbing light energy and it moves to a higher energy level.
Energy of a photon = Energy difference between the ground state and an excited state
h = Planck’s constant (6.63x10-34 J.sec.)
u = frequency of light
E = energy of photon (measured with UV. or visible spectroscopy)
MAGNETIC PROPERTIES OF METAL COMPLEXES
- Paramagnetic - unpaired electrons
- Diamagnetic - no unpaired electrons
- Determined from crystal field splitting diagrams
STABILITY OF COORDINATION COMPOUNDS IN SOLUTION
Consider the following equilibrium between undissociated complex ion and dissociated ion.
[MLn]b+ 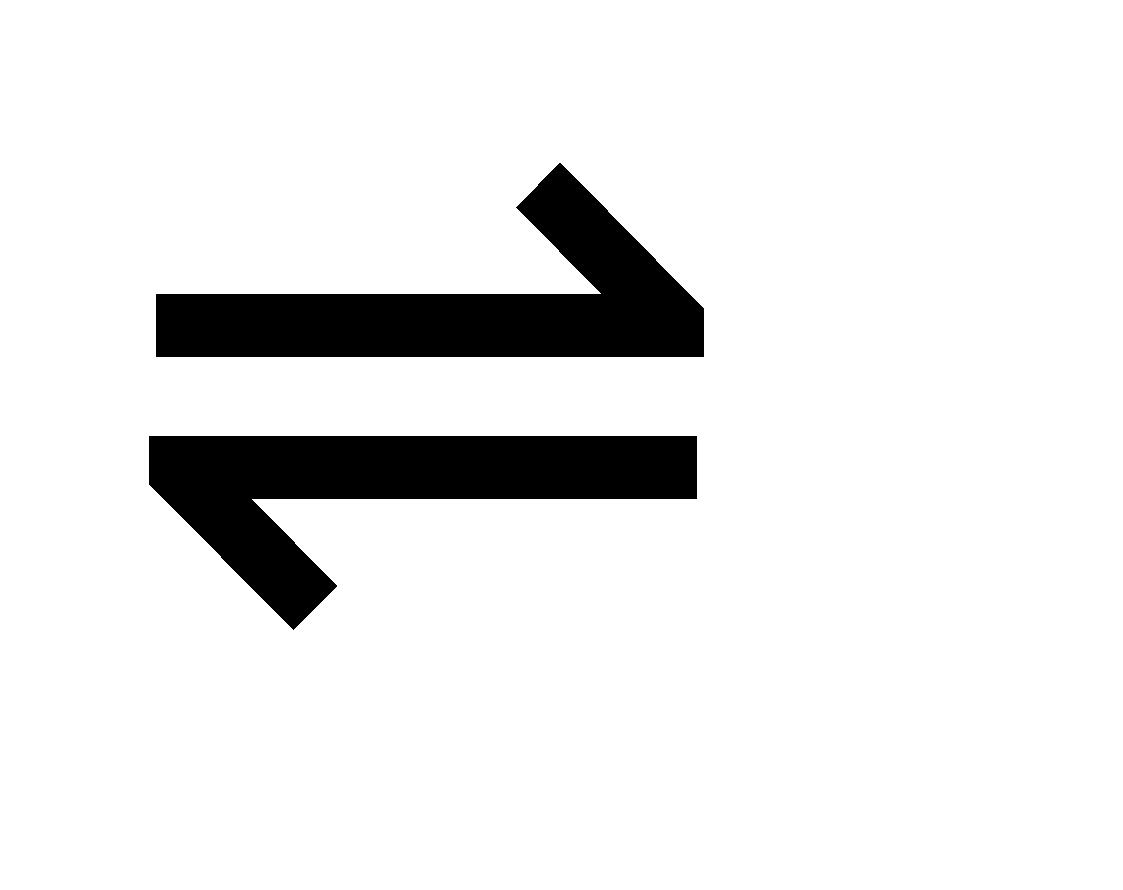 Ma+ +nLx-
Ma+ +nLx-
The equilibrium constant Kc = 
The smaller the value of Kc, the greater is the stability of complex ion and vice versa.
The reciprocal of equilibrium constant is called stability constant.
The reciprocal of equilibrium constant is called stability constant.
The higher the value of Ks, the more is the stability of complex ion. The value of Ks depends on.
- Nature of central metal atom - The more the polarizing power of the central metal ion the more is the stability of complex ion.
The polarising power  =
= 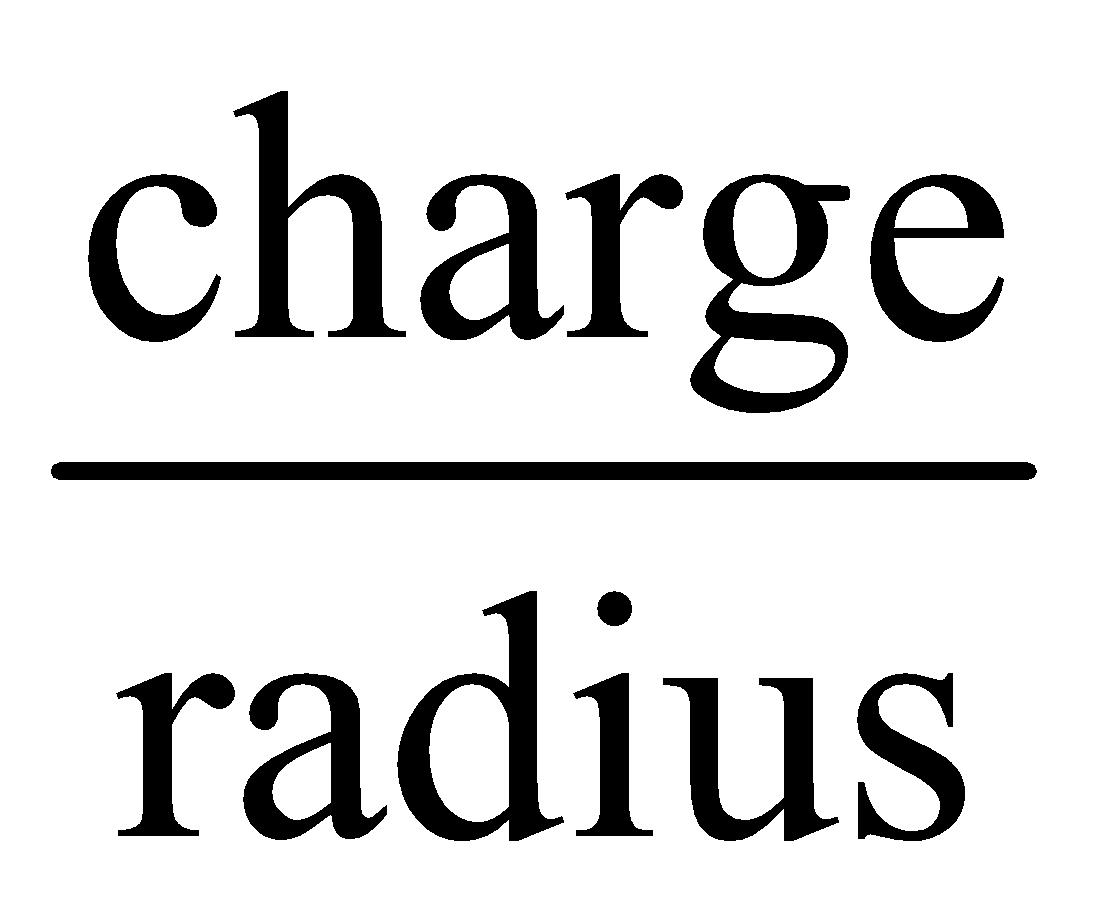
Thus complex of Fe3+ is more stable than Fe2+
- Nature of ligand - Since ligand is a Lewis base the more the basic character of ligand the more is the stability of complex ion. Thus complex ion of F–is more stable than that of
Chelating ligands give much larger values of stability constant.
Perfect or penetrating complexes
They are fairly stable and dissociate negligibly or not at all e.g.
They are fairly stable and dissociate negligibly or not at all e.g.
Imperfect or normal complexes
The complex ion is reversibly dissociated
IMPORTANCE OF COORDINATION COMPOUNDS
BIOLOGICAL PROCESSES
Haemoglobin - Oxygen carrier is a complex of Iron (II)
Chlorophyll - Green colouring matter of plants is a complex of Mg
Vitamin B12 - It is a complex of cobalt
ANALYTICAL CHEMISTRY
Many metal ions are quantitatively estimated by complex formation eg Cu++, Ni2+, Fe3+, Al3+
Red precipitate of nickel with dimethylglyoxime
Separation of Ag+ and, Ag+ form soluble complex
AgCl + 2NH4OH  [Ag (NH3)2]Cl + 2H2O
[Ag (NH3)2]Cl + 2H2O
Soluble complex
METALLURGICAL PROCESSES
- Bauxite ore of aluminium is purified by soluble complex formation
Impurities of Fe2O3 are left behind in solution.
- Extraction of silver and gold by cyanide process involves complex formation
- Nickel is purified by Mond’s process forming volatile nickel carbonyl
PHOTOGRAPHY
Excess of AgBr is removed by complex formation
AgBr(s) + 3 Na2S2O3 (aq)  Na3 [Ag(S2O3)2] (aq)+ NaBr (aq)
Na3 [Ag(S2O3)2] (aq)+ NaBr (aq)
MISCELLANEOUS USES
- K [Ag(CN)2] complex of silver is used in silver plating
- EDTA (ethylene diamine tetra acetate) is used for the estimation of Mg2+ and Ca2+ ions and for removal of hardness of water
- [Pt(NH3)2Cl2] known as cisplatin is used in the treatment of cancer
ORGANOMETALLIC COMPOUNDS
The organic compounds having metal atom directly attached to the carbon are known as organometallic compounds.
𝝈 - bonded
R - Mg - X Alkyl magnesium halide commonly known as Grignard’s reagent
R - Mg - X Alkyl magnesium halide commonly known as Grignard’s reagent
π - bonded
(CH3)4 Sn (Tetramethyl tin), (C2H5)2 Zn (Diethyl Zinc), n–C4H9Li (n–butyllithium)
K [PtCl3–h2 –(C2H4)] (Zeise’s salt)
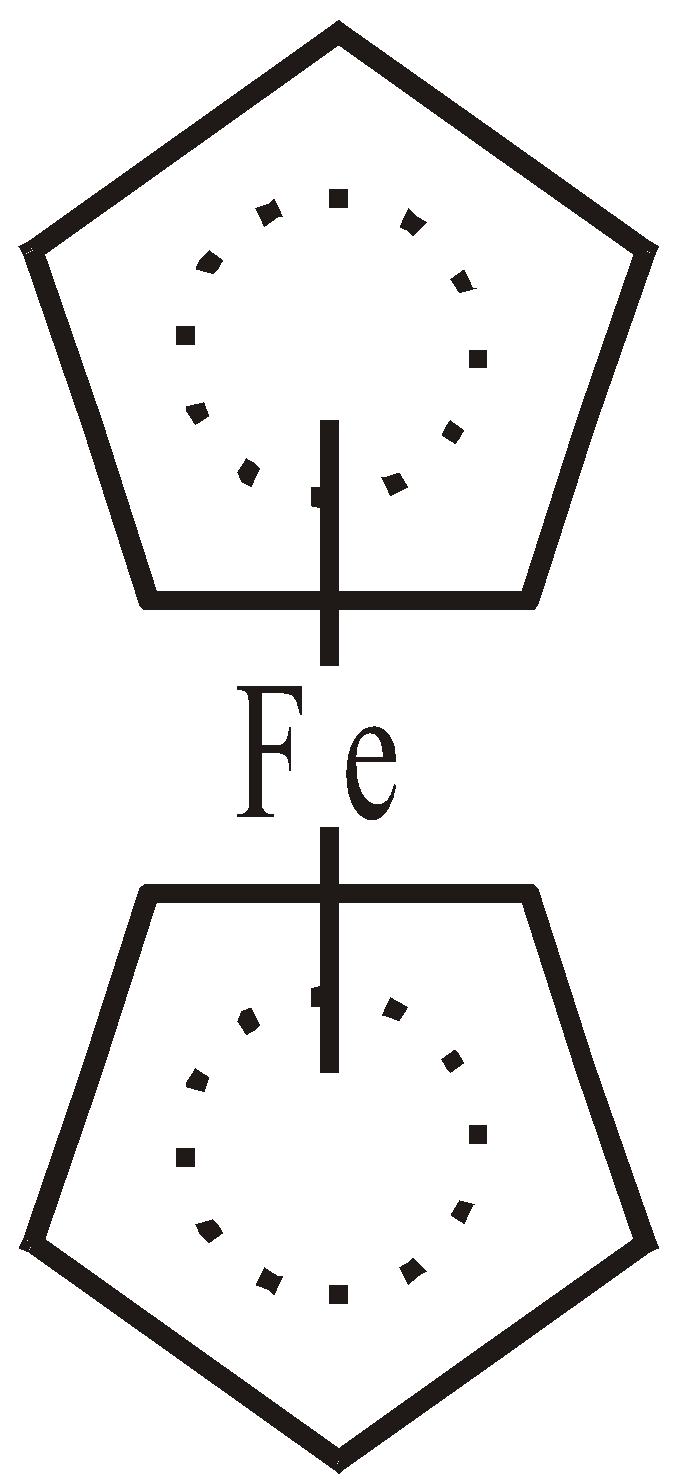
Fe[h5– C5H5]2 Ferrocene
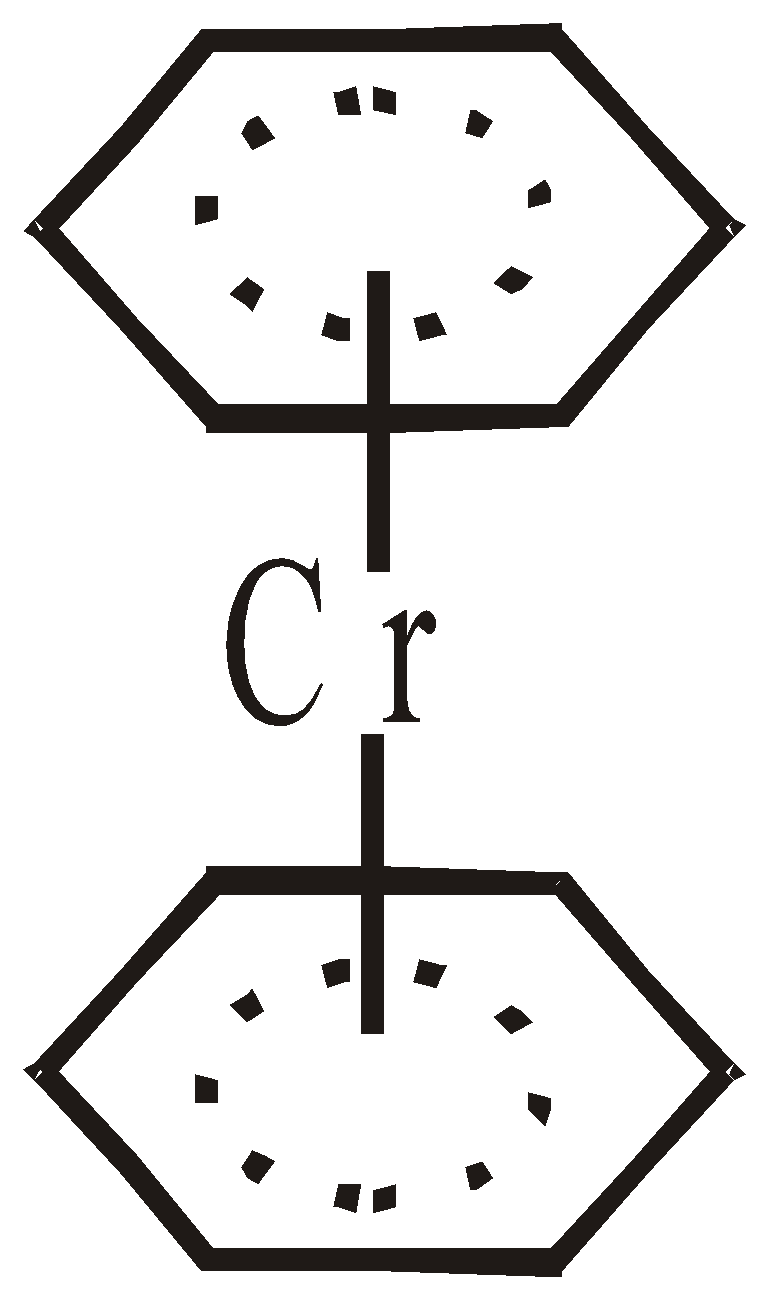
Cr[h6– C6H6]2 Dibenzene chromium
SYNTHESIS OF ORGANOMETALLIC COMPOUNDS
SYNTHESIS OF GRIGNARD’S REAGENT
By reaction between an alkyl halide and Mg in presence of ether
By reaction between an alkyl halide and Mg in presence of ether
Other metals like Li, Na, Zn, Cd can also be used.
SYNTHESIS OF OTHER ORGANOMETALLIC COMPOUNDS USING GRIGNARD'S REAGENT
PREPARATION OF P COMPLEXES
Zeise’s salt - 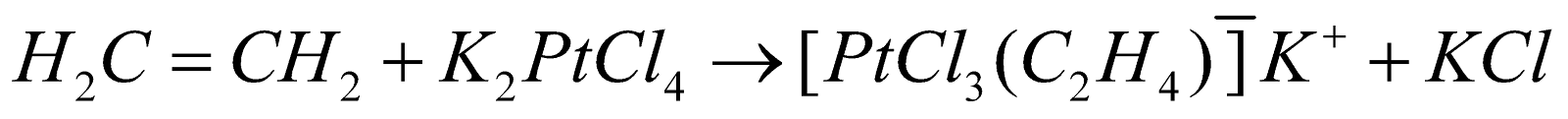
Dibenzene chromium - 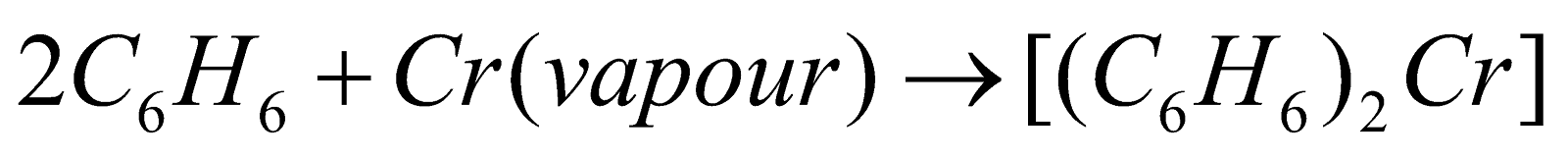
Ferrocene - 
METAL CARBONYLS
The compounds of carbon monoxide with certain transition metals are known as metal carbonyls
Polynuclear metal carbonyls are also known e.g. Fe3(CO)12 Mn2(CO)10
The metal carbon bond in carbonyls may be represented as M⇠ C☰O. Due to some back bonding by sidewise overlapping between d orbitals of metal and empty p-orbitals of carbons, the M–C bond length is somewhat shorter and C–O bond is longer than triple bond.
Preparation of metal carbonyls
By passing CO overheated metal eg.
By passing CO overheated metal eg.
USES OF ORGANOMETALLIC COMPOUNDS
- Grignard’s reagent is employed for the synthesis of number of organic compounds eg alcohols, aldehydes, ketones, esters etc.
- Nickel is purified by Mond's, process forming Ni(CO)4.
- Ziegler Natta Catalyst which is mixture of triethylaluminum and titanium Chloride (Al (C2H5)3 + TiCl3) is used for polymerisation of ethene.
- Tetraethyl lead is used as antiknock compound Pb(C2H5)4 .
- Wilkinson’s catalyst (Ph3P)3 RhCl. for selective hydrogenation .
- C2H5HgCl (ethyl mercury chloride) as fungicide.










