d- AND f-BLOCK ELEMENTS
TRANSITION ELEMENTS
Elements where the last orbitals filled are the d orbitals known as transition elements. They have been placed in the middle of the periodic table between electropositive s-block and electronegative p-block elements.
GENERAL ELECTRONIC CONFIGURATION
Transition metals have the electronic configuration (n-1)d1-10ns0-2. When electrons fill orbitals, ns-orbital is filled first than (n-1)d-orbital.When losing during oxidation, ns electrons are lost first than (n-1)d electrons.
Zn ,Cd, Hg ,the end members of first three series have their general electronic configuration (n-1)d10ns2. These do not show properties of transition elements to any appreciable extent and are called non-typical transition elements.
CLASSIFICATION
Transition elements consist of the following four series
ELECTRONIC CONFIGURATION OF TRANSITION ELEMENTS
PHYSICAL PROPERTIES OF TRANSITION ELEMENTS
METALLIC CHARACTER
Transition metals can lose valence electrons and form cations 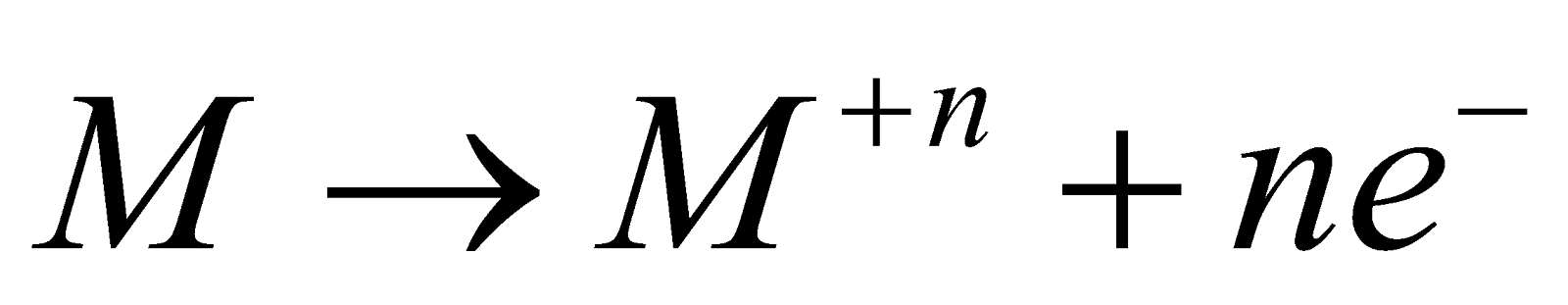
They have simple hcp, ccp and bcc lattices characteristic of true metals. Except Hg, they are solids at room temperature and are dense ( in general, in case of osmium 22.6g/), lustrous, malleable, ductile thermal and electrical conductors. There is gradual decrease in electropositive character from left to right.
in general, in case of osmium 22.6g/), lustrous, malleable, ductile thermal and electrical conductors. There is gradual decrease in electropositive character from left to right.
MELTING AND BOILING POINT
Due to strong metallic bond, they have high mpts and bpts. The mpts of these elements rise to a maximum and then fall with the increase in atomic number the manganese and technetium show abnormal values as shown by graph)
IONISATION ENERGY
The ionisation energy increases with the increase in the atomic number but not in regular manner. The ionisation energies of 5d elements are higher than those of 4 d and 3d elements due to greater effective nuclear charge which in turn is due to poor shielding of nucleus by 4f electrons.
Formation of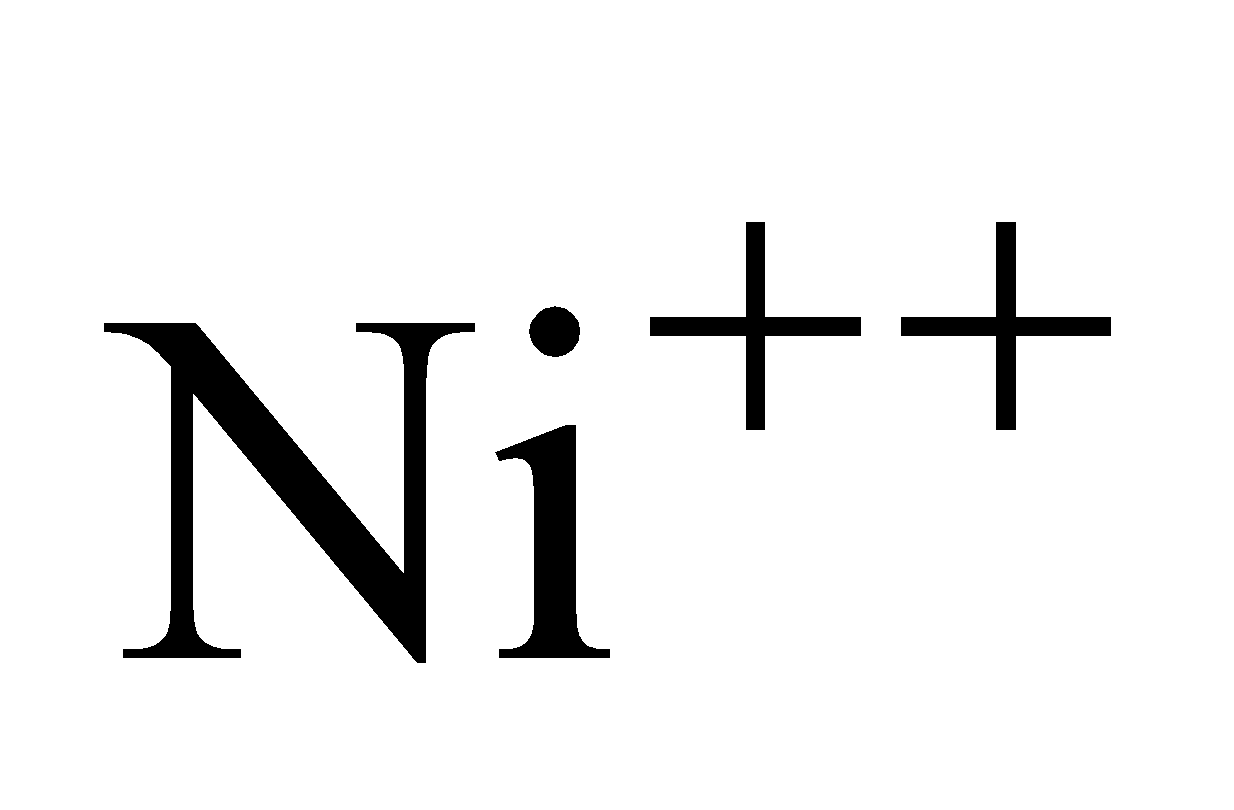 requires
requires 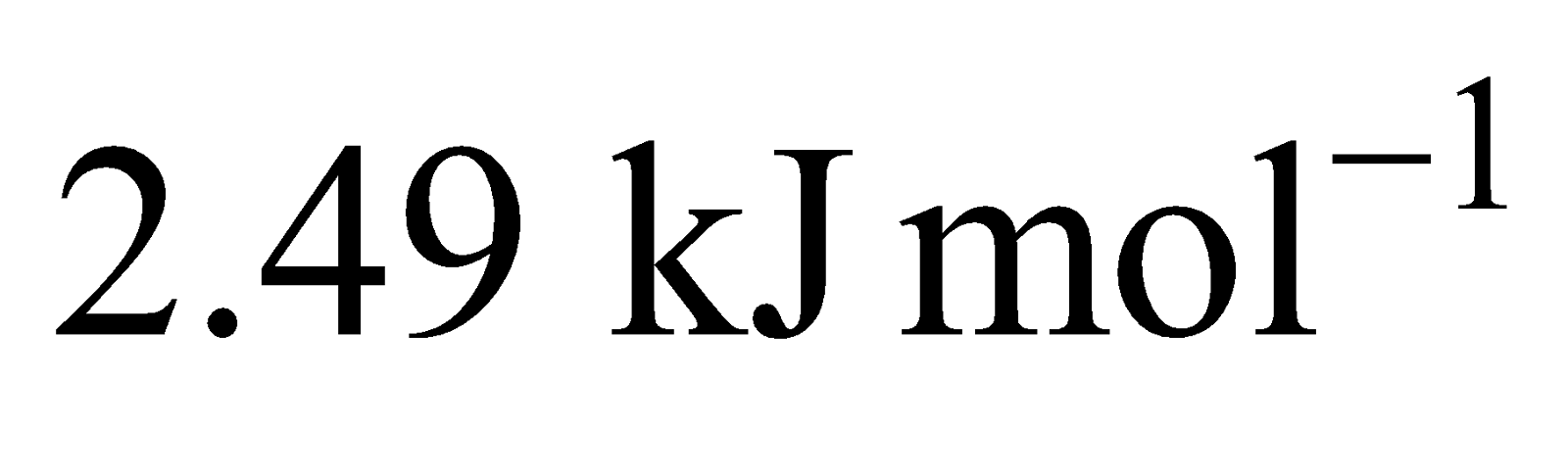 and formation
and formation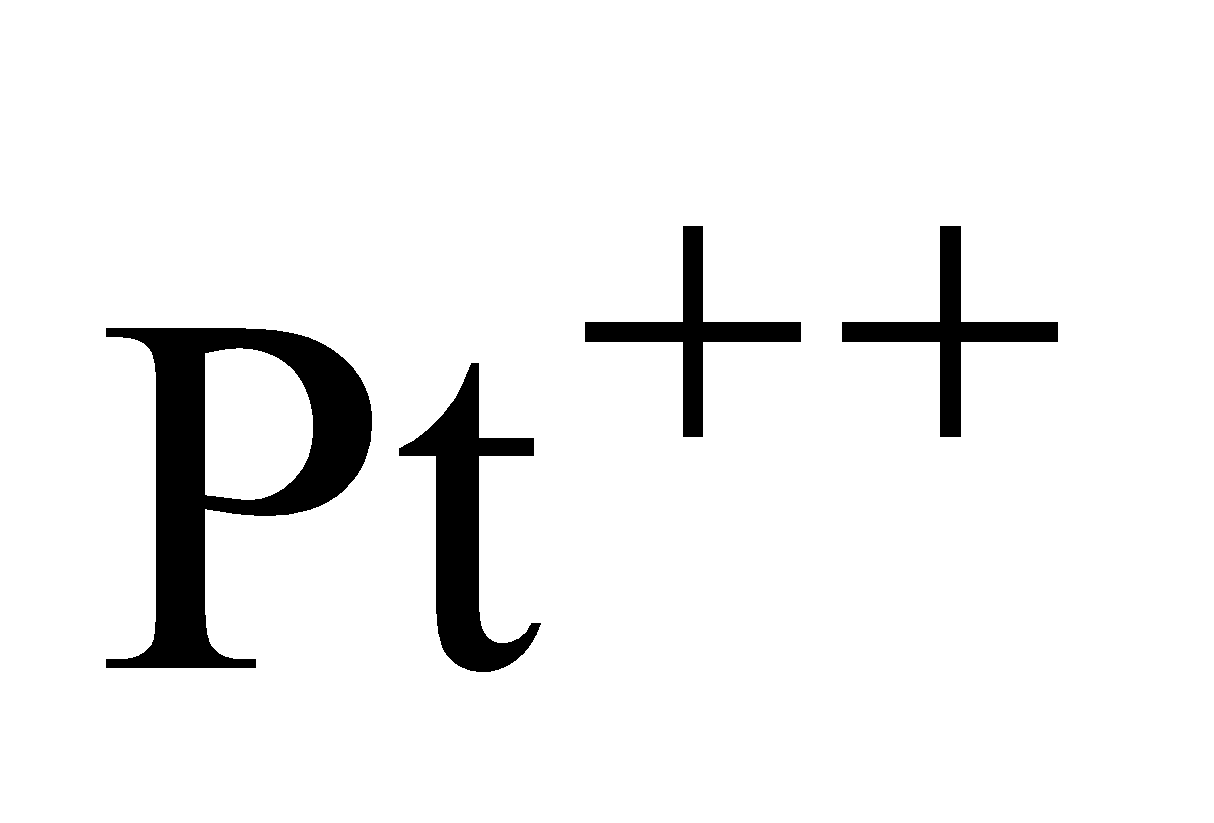 requires
requires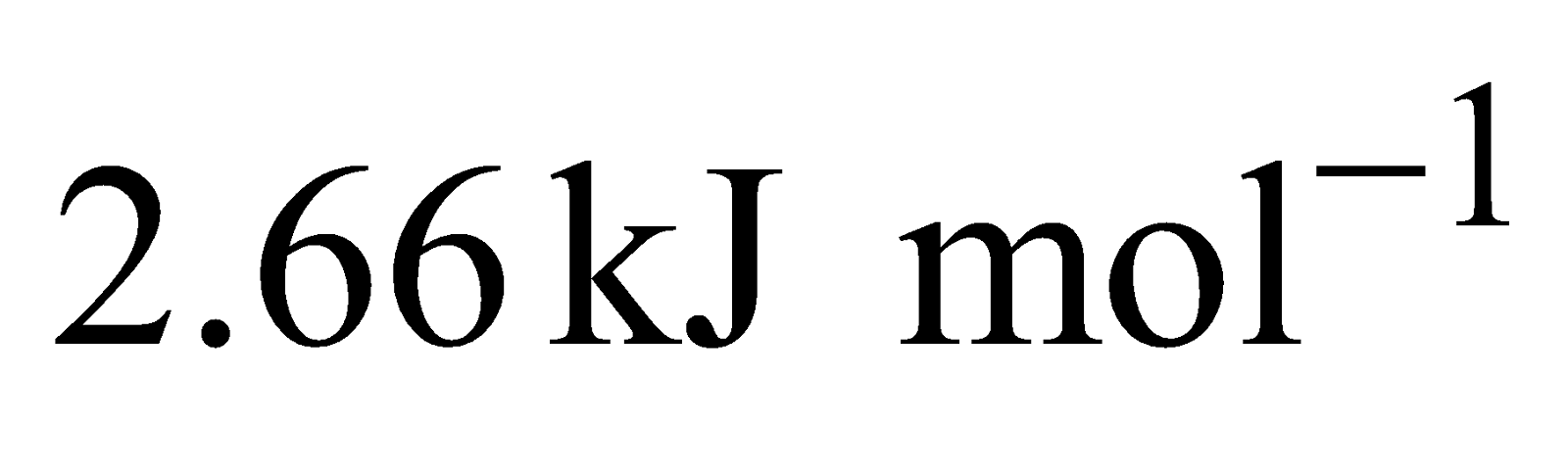 .
.
Hence Ni (II) compounds are thermodynamically more stable than Pt (II) Compounds.
Formation of requires
requires  and formation of
and formation of  requires
requires
Hence Pt (IV) compounds are relatively more stable than nickel (IV) compounds.
Thus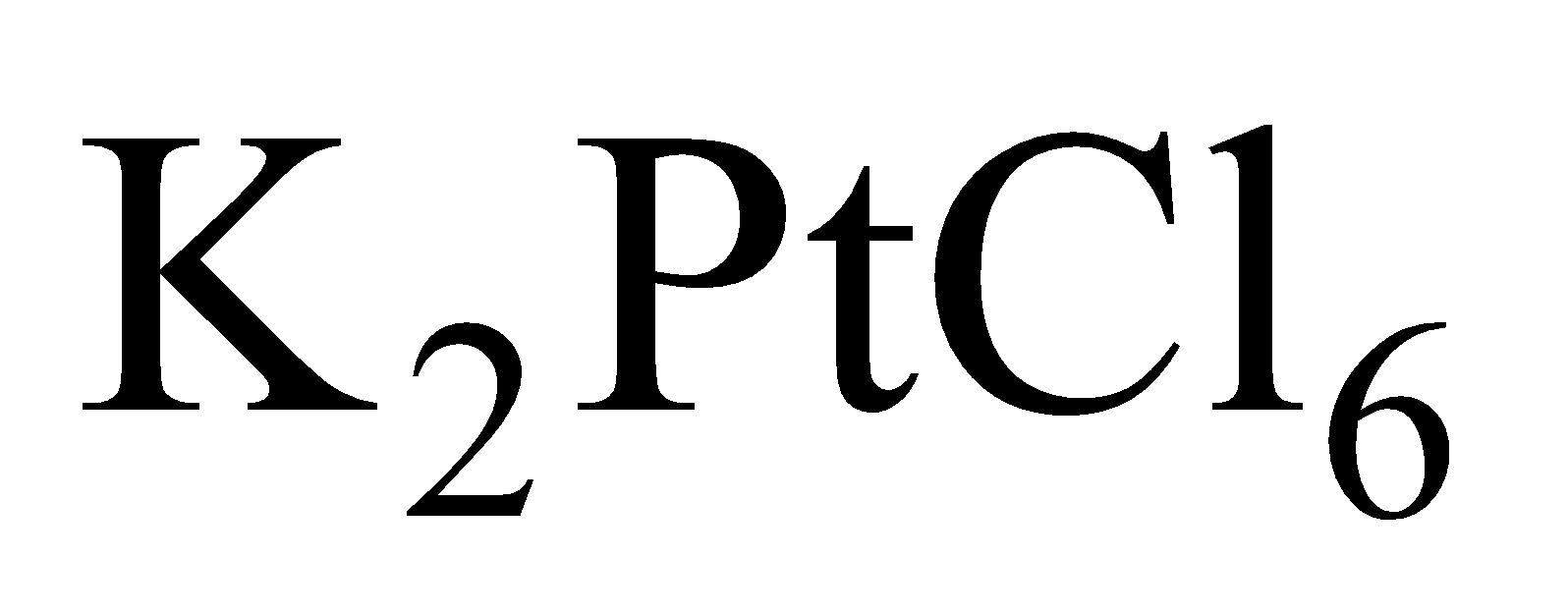 is well known where as the corresponding nickel compound is not known. (Ionisation energy graph is sketched here for ready reference)
is well known where as the corresponding nickel compound is not known. (Ionisation energy graph is sketched here for ready reference)
ELECTRODE POTENTIAL
(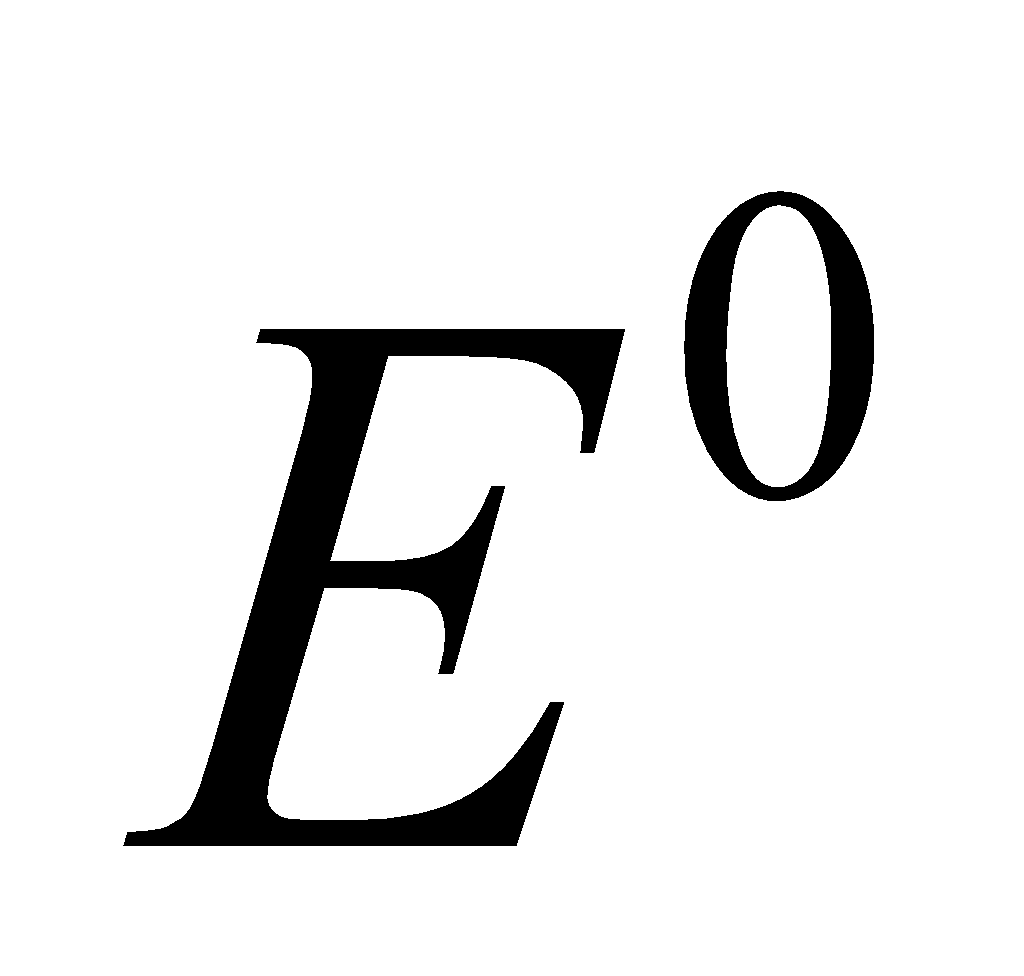 ) (
) (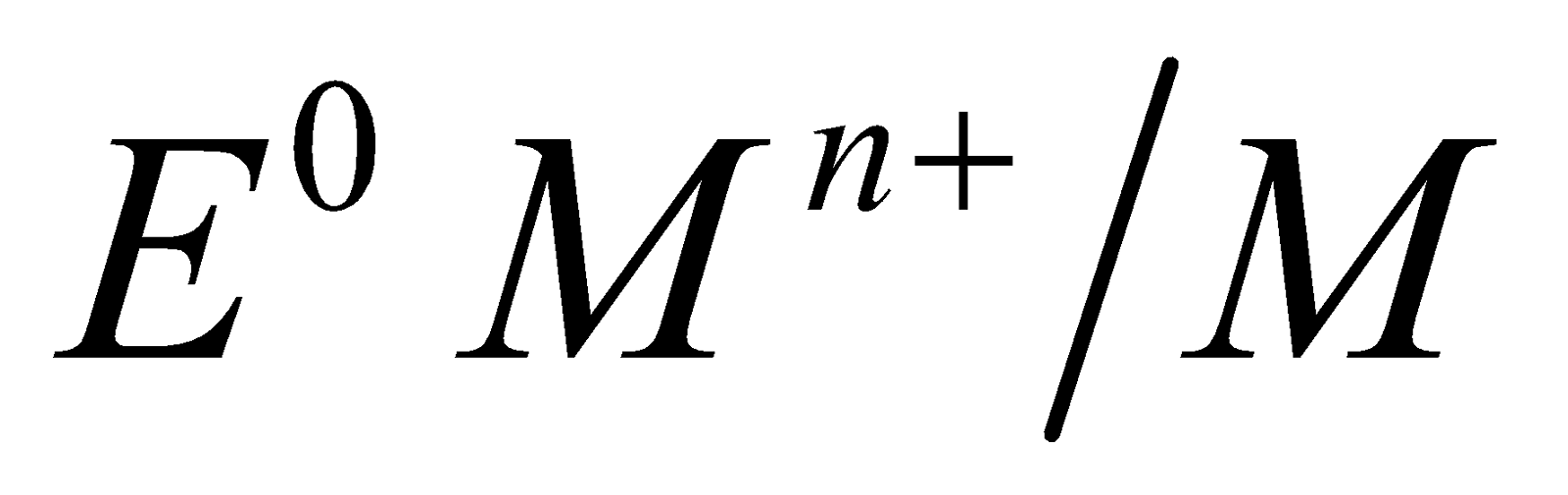 ) is governed by three factors
) is governed by three factors
- Heat of sublimation
- Heat of ionisation
- Heat of hydration
For the 3d transition metals the values are
V Cr Mn Fe Co Ni Cu
-1.18 -0.91 -1.18 -0.44 -0.28 -0.25 0.35 (Volts)
The irregular trend is due to variation in ionization energies and sublimation energies. Except copper 3d elements are good reducing agents but weaker than s-block elements.
OXIDATION STATES
In different types of compounds , transition metals exhibit different oxidation states. The highest oxidation state is exhibited in fluorides and oxides. In lower oxidation state the compounds formed are ionic and in higher oxidation state they are covalent in nature.
Osmium exhibit +8 O.S. (highest)often but Ru exhibit +8 oxidation state rarely. Transition metals also show oxidation states +1 and zero.
Fe3+ is more stable than Fe2+. Hence Fe2+ act as reducing agent Cr3+ is more stable than Cr2+. Hence Cr2+ act as reducing. Mn2+ is more stable than Mn3+ Hence Mn3+ act as oxidising agent
ATOMIC AND IONIC RADII
The values for atomic radii and ionic radii are in between the values for s and p-block elements. In 3d transition series the ionic radii for ion decreases upto the middle of the period then becomes almost constant. Due to lanthanide contraction the second and third member of each group have atomic radii close to each other (Zr.160pm, Hf 159pm)
ion decreases upto the middle of the period then becomes almost constant. Due to lanthanide contraction the second and third member of each group have atomic radii close to each other (Zr.160pm, Hf 159pm)
DENSITY
d-block elements have high density because of their small atomic sizes and strong metallic bonding.
Density Sc Ti V Cr Mn Fe Co Ni Cu Zn
g/ml 3.0 4.54 6.10 7.19 7.40 7.87 8.70 8.90 8.92 7 .13
ATOMIC VOLUME
Atomic volume decreases along the period due to decrease in size.
REACTIVITY
d-block elements are less reactive due to high ionisation energies. Some are almost inert and known as noble metals, e.g. Au, Pt, Ru, Rh, Os, Ir etc.
COMPLEX FORMATION
They are well known to form a large number of complex compounds mainly due to
- Small atomic size and higher nuclear charge
- Presence of partly filled or vacant orbitals
eg.
COLOURED IONS
The colour exhibited by transition metal ions is due to the presence of unpaired electrons in d-orbitals which permits the d-d excitation of electrons.
Colour of a complex depends on the metal, its oxidation state and its ligands. e.g.
[ Cu(H2O)4 ]2+ is Pale blue
[ Cu(NH3)4 ]2+ is Dark blue
CuSO4.5H2O is blue in colour and anhydrous CuSO4 is colourless.
In absence of ligands all d orbitals are degenerate (same energy) and the possibility of d-d excitation is no more.
In presence of ligand  have higher energy, d-d transition take place by absorption of light, hence the colour.
have higher energy, d-d transition take place by absorption of light, hence the colour.
MAGNETIC PROPERTIES
- Paramagnetic - This is due to the presence of unpaired electrons in d-orbitals. Paramagnetic character increases with the number of unpaired electrons.
- Diamagnetic - Diamagnetic substances are repelled by an applied magnetic field.
- Ferromagnetism - In this case permanent magnetic moment is acquired by substance e.g. Fe. Magnetic moment is given by
B.M. where n = number of unpaired electrons and B.M. = Bohr magneton (unit of magnetic moment)
CATALYTIC PROPERTIES
The transition metals and their compounds behave as catalyst due to-
- The presence of partly filled d-orbitals and exhibiting various oxidation states.
- Their formation of intermediate complex with reactants and thus lowering the energy of activation
- Their rough surface area provides active sites for adsorption of reactant molecules. eg.
Iron in the preparation of NH3 (Habers process)
Finely divided nickel for hydrogenation
Pt or V2O5 in the preparation of H2SO4 (Contact process)
Pt in the preparation of nitric acid (Ostwald’s process)
FORMATION OF ALLOYS
d block elements have a strong tendency to form alloys since their atomic sizes are very similar and in the crystal lattice one metal can be readily replaced by another. Alloys so formed are hard, have high m.pts. The metals Mo, W, Cr, Ni, and V are used for the production of stainless steel and alloy steel.
Amalgam is an alloy formed by mercury with other metals. Iron and platinum do not form any alloy with mercury.
INTERSTITIAL COMPOUNDS
The empty space present in a crystal lattice is known as interstitial place. The non metal atoms due to their small size (eg H, B, N, C etc.) when occupy such place the resulting compound is known interstitial compound. Such compounds are hard and rigid e.g. cast iron and steel.
NON STOICHIOMETRIC COMPOUNDS
The compounds not having the elements in the exact ratio as shown by the molecular formula are known as non stoichiometric compounds e.g., etc. In FeO the Fe:O is approx. 0.94:1 and not exactly 1:1.
IRON 
OCCURRENCE
Being reactive in nature it does not occur in free state.
Ores of Iron-
- Haematite
- Magnetite
- Limonite or hydrated ferric oxide
- Iron pyrites
- Siderite
- Copper pyrites
EXTRACTION
It is extracted from haematite 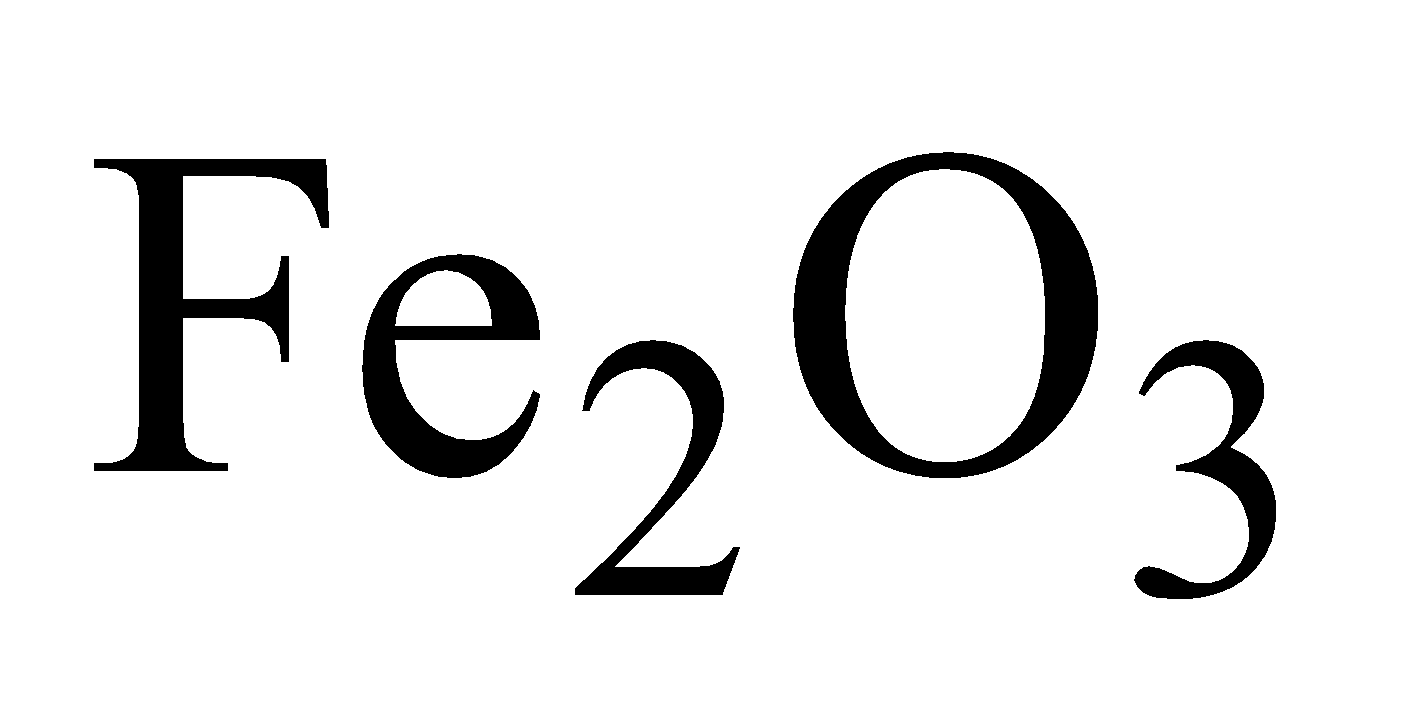 in a blast furnace by reduction with carbon and carbon monoxide. The steps involved are-
in a blast furnace by reduction with carbon and carbon monoxide. The steps involved are-
- Concentration - The crushed ore is agitated with water and then concentrated by electromagnetic method.
- Roasting or Calcination - To remove volatile substances and organic matter.
- Smelting - Roasted or calcinated ore is mixed with limestone and coke and fed into blast furnace. Reactions taking place in the blast furnace.
Lower region

Middle region
Upper region
The gases leaving the furnace contain CO and used to heat incoming air blast.The two layers in the blast furnace are-
Upper layer - Molten Iron - It is poured out in moulds and known as PIG IRON or CAST IRON.It contains 3-5% carbon and varying amounts of Mn, Si, P and S which make the iron hard and brittle.
Lower layer - Molten CaSiO3 (slag)
WROUGHT IRON
It is obtained by heating cast iron with haematite. The impurities are oxidised.
It contains carbon 0.2-0.5% and traces of P and Si. It is pure form of Iron and soft , malleable, ductile. It is used to make magnets in electric cranes and dynamos, railway carriage couplings being corrosion resistant.
STEEL
It contains carbon 0.1-1.5% and manufactured by following methods.
- Bessemer process - Molten pig iron is heated in large pear shaped furnace lined with silica bricks at 1873K when impurities such as Mn, Si, C burn off. When all carbon is completely burn off the requisite amount of carbon is added.
Bessemer converter is lined with lime (CaO) or magnesia (MgO) when pig iron contains high percentage of phosphorous.
- Open hearth process - The cast iron, scrap iron , haematite ore and lime are mixed together and melted in open hearth furnace lined with or calcined dolomite (MgO. CaO) depending upon the nature of impurities.
- Electric furnace process - It is combination of Bessemer and open hearth process.
TYPES OF STEEL
- Soft steel - contains carbon 0.25%
- Mild steel - contains carbon 0.25-0.5%
- Hard steel - contains carbon 0.5-1.5%
- Alloy steel - contains varying percentage of Ni, Cr, Mn, Co,W, V e.g. stainless steel is an alloy of Fe, Cr and Ni.
HEAT TREATMENT OF STEEL
The hardness of steel depends on its carbon content and heat treatment.
The hardness of steel depends on its carbon content and heat treatment.
- Quenching - It involves the heating of steel to red hot (1123K) and cooling it by plunging into cold water or oil. It makes the steel hard and brittle.
- Annealing - The steel is heated well below red heat and then cooled slowly. The steel becomes soft.
- Tempering - In this process the quenched steel is reheated to 504 to 574K and allowed to cool slowly. The brittleness disappears and hardness is retained.
- Nitriding - It involves the heating of steel in an atmosphere of ammonia when surface is coated with iron nitride. The steel becomes hard.
- Case hardening -The steel is heated in charcoal and then quenched.The steel becomes hard.
ALLOYS OF STEEL
The important alloy steels are-
The important alloy steels are-
Name
|
Composition
|
Uses and Properties
|
Tungsten steel
|
Fe 94%, W 5%, C 1%
|
It is very hard, resistant to water and used for making Rock drills and Safeties
|
Stainless steel
|
Fe 73% Cr 18%, Ni 8%, C 1%
|
It is resistant to corrosion. Used for making cutlery
|
Manganese steel
|
Fe 86%, Mn 13%, C 1%
|
It is hard, used for manufacturing high speed cutting tools
|
Invar
|
Fe 64%, Ni 36%
|
It has small coefficient of expansion, used in watches, meter scales and pendulum rods
|
Permalloy
|
Fe 21%, Ni 78%, C 1%
|
It is strongly magnetised by electric current and lose magnetism when current is let off, used for manufacturing electromagnets and ocean cable.
|
Nickel steel
|
Fe 96-98%, Ni 2-4%
|
Resistant to corrosion, hard and elastic wire, used for making cables, gears and drive shafts.
|
SOME CHEMICAL PROPERTIES OF IRON
- Red hot iron burns in O2 giving sparks
- When steam is passed over red hot iron, hydrogen is liberated and magnetic oxide of iron (ferroso ferric oxide) is formed
- Action of dil. H2SO4
- Hot and conc. H2SO4
- Action of dil. HNO3
PASSIVITY
The inertness exhibited by metals under conditions when chemical activity is to be expected is called passivity. Iron becomes passive with conc. HNO3, Chromic acid, conc. H2SO4 and KMnO4 etc. It is due to the formation of a thin layer of oxide at the surface of iron.
COMPOUNDS OF IRON
FERRIC CHLORIDE
PREPARATION
- By passing dry chlorine over heated iron, anhydrous ferric chloride is obtained
- By the action of hydrochloric acid on ferric hydroxide or ferric oxide
PROPERTIES
- It is soluble in water, alcohol and ether.
is yellow. Its aqueous solution is acidic. Sublimes at 300ºC, covalent and dissociates above 973K first into
and
e.g.
- Action of heat
- Oxidising nature of FeCl3 is shown by following reactions
STRUCTURE
USES
Used in medicine as ASTRINGENT and ANTISEPTIC. Its concentrated solution is used for etching copper and silver.
FERROUS SULPHATE (GREEN VITRIOL)
PREPARATION
- Manufacture - From iron pyrites by oxidation by air
PROPERTIES
- Hydrated
is green and anhydrous is colourless.
- Action of heat
- Its aqueous solution is acidic due to cationic hydrolysis
- Reducing nature - It is strong reducing is nature.
- Addition compound with NO which is dark brown
USES
As mordant in dyeing, insecticide and in the preparation of Mohr’s salt.
FERROUS AMMONIUM SULPHATE (MOHR’S SALT)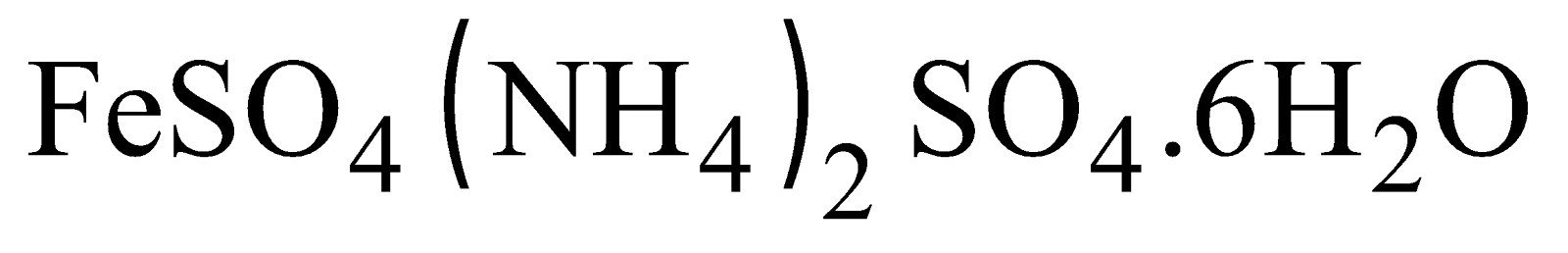
PREPARATION
By mixing saturated solutions of and cooling.
and cooling.
By mixing saturated solutions of
PROPERTIES
It is light green crystalline compound and does not effloresce.
It is light green crystalline compound and does not effloresce.
FERRIC OXIDE
In nature it occurs as haematite.
Used in Bosch process as catalyst and polishing powder by jewellers and as red pigment.
IRON SULPHIDE FeS
By heating iron filings with dil. H2SO4
The reaction is carried out in Kipp’s apparatus. FeSO4 is obtained as by product H2S. Finds an extensive application in analytical chemistry. It has smell of rotten eggs.
COPPER
OCCURRENCE
It occurs in nature in large quantities in Michigan (USA).
Important ores are
- Copper glance
- Copper pyrites
- Malachite
- Cuprite
(Ruby copper)
- Azurite
PREPARATION
EXTRACTION
It is mainly extracted from copper pyrites.
CONCENTRATION
The ore is concentrated by froth floatation process.
ROASTING
The concentrated ore is strongly heated by hot blast of air on the hearth of reverberatory furnace. The following changes take place
SMELTING
The roasted ore is mixed with sand and heated in blast furnace.
The mixture of copper and iron sulphides melt together to form “matte”.
BESSEMERISATION
The molten matte mixed with little sand is poured into Bessemer converter. The following changes take place
The copper thus produced is called ‘blister copper’ and contains 2.0% impurities of Ag, Au, Ni, Zn, Pb, Sn, As, S etc.
REFINING
It is carried out by either of the following methods
- By polling - The melt is stirred vigorously with green poles of wood and oxides are reduced by hydrocarbons emanating from wood.
- Electrolytic refining of copper - Slabs of impure copper are made anode and thin sheets of pure copper as the cathode. Acidic copper sulphate is used as electrolyte.
The impurities like Zn, Fe, Ni,Co remain in solution being more electropositive in nature and Ag, Au, Pt, (less electropositive) collect below the anode in the form of anode mud or slime 99.99% pure copper is obtained. Anode mud provides about 25% of U.S. Silver production and 13% of U.S. gold production.
PROPERTIES OF COPPER
In aqueous solution it has two oxidation states +1 (cuprous) and +2 (cupric). Cu (I) salts tend to be white and insoluble in water while many salts of Cu (II) are water soluble however Cu(OH)2 is insoluble. CuS is one of the least soluble compounds.
- Cu (I) disproportionates easily in aqueous solution.
- Action of
- It is attacked by
first forming copper (I) oxide
(Red) and then copper (II) oxide CuO (Black).
- It forms a green layer of basic carbonate in presence of
and moisture.
- Not attacked by dilute acids e.g. HCl and but dissolves in these acids in presence of air.
COMPOUNDS OF COPPER
COPPER SULPHATE (BLUE VITRIOL OR NILA THOTHA)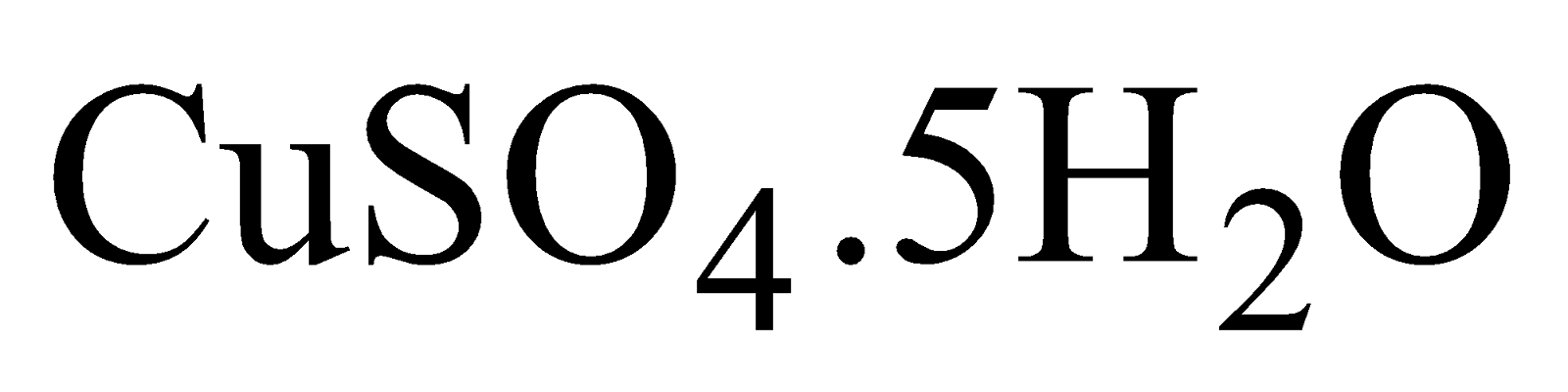
PREPARATION
- By dissolving Cu (II) oxide or carbonate in dilute
.
- From scrap copper
PROPERTIES
- Blue crystalline compound.
- Action of heat
- Action of NH4OH
Tetrammine copper sulphate is known as SCHWITZER’S REAGENT. It is used to dissolve cellulose in the manufacture of artificial silk.
- Action of KI
(It does not react with KCl, KBr or KF)
- Action of potassium ferrocyanide
- Action of KCN
- Structure of
Four with cation and fifth with anion.
USES
In electroplating, as mordant in dyeing.
Bordeaux mixture (Mixture of 
It is used as fungicide. In the preparation of Fehling solution and electric batteries.
It is used as fungicide. In the preparation of Fehling solution and electric batteries.
CUPROUS CHLORIDE - COPPER (I) CHLORIDE 
PREPARATION
- It can be prepared from Cu alone or in combination with by action of concentrated hydrochloric acid.
- By passing in a solution of
PROPERTIES
- It is a white solid, almost insoluble in water.
- Action of conc. HCl - It dissolves forming soluble complex.
or 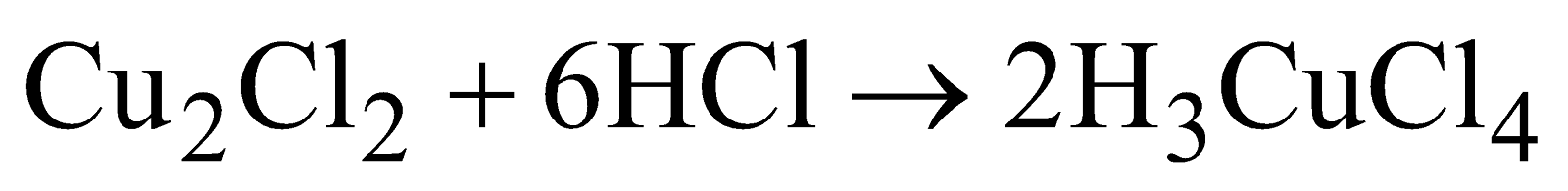
On dilution white precipitate again appears.
- Action of ammonia - It dissolves forming soluble complex
- Action of acetylene - Red precipitate of cuprous acetylide is obtained.
- With carbon monoxide it forms addition product.
- With air - In air it is slowly oxidised to green basic cupric chloride.
- With NaOH
- With H2S
USES
In gas analysis for absorbing 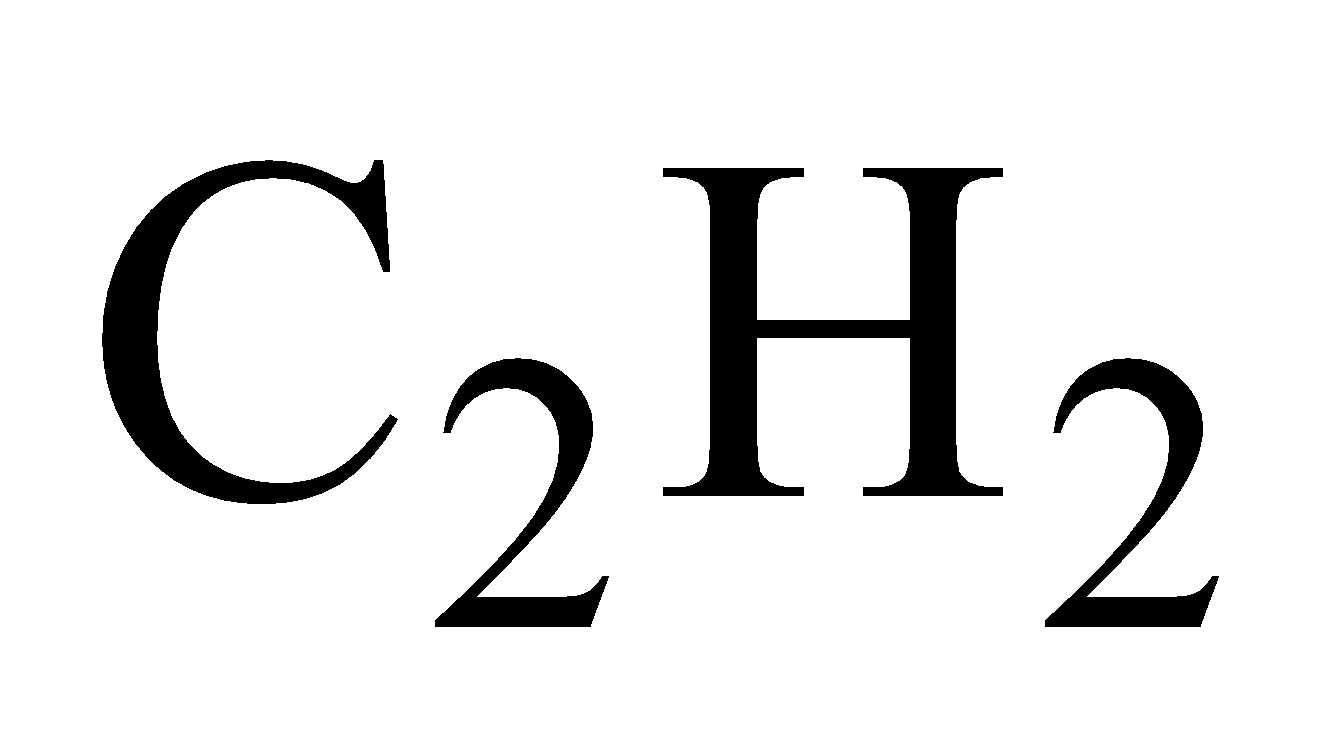 and CO. In combination with
and CO. In combination with  as catalyst for synthetic rubber.
as catalyst for synthetic rubber.
CUPRIC CHLORIDE COPPER (II) CHLORIDE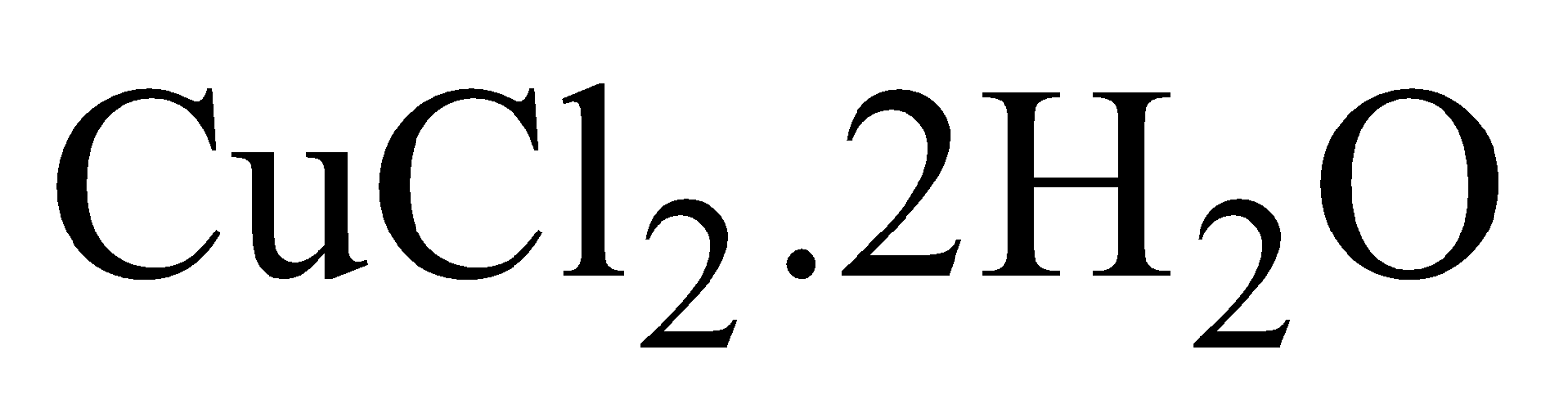
PREPARATION
- Form copper, cupric oxide or copper carbonate by the action of conc. HCl.
- Anhydrous cupric chloride is prepared by burning copper in current of chlorine
PROPERTIES
- Hydrated
- Anhydrous
- Aqueous dilute solution is blue due to complex
- Concentrated solution is green due to complex
- With Ammonia - First a precipitate which dissolves in excess of
.
- Action of heat
- Hydrated salt on heating gives
STRUCTURE
CUPROUS OXIDE (RED OXIDE OF COPPER)
PREPARATION
When Fehling solution is reduced by glucose or aldehyde.
PROPERTIES
Red colour, insoluble in water. It forms stable complexes.
USES
In making ruby red glass and enamel.In manufacturing anti rust paints.
CUPRIC OXIDE (BLACK OXIDE OF COPPER)
PREPARATION
By heating malachite which is native copper carbonate.
PROPERTIES
Black powder reduced to metallic copper by .
.
USES
In the manufacture of glass.It gives green colour to glazes and glass.
SILVER (Ag)
OCCURRENCE
It is found in nature and in combined state.
Principal ores are -
- Argentite (silver glance)
- Horn silver AgCl,
- Pyrargyrite (ruby silver)
In small quantities in lead ,copper and zinc ores.
EXTRACTION
MAC ARTHER FOREST’S CYANIDE PROCESS
- Concentration - Ore is concentrated by froth floatation process.
- Treatment with NaCN - The powdered ore is treated with NaCN solution (0.7%) and air is bubbled through the mixture.
(a) 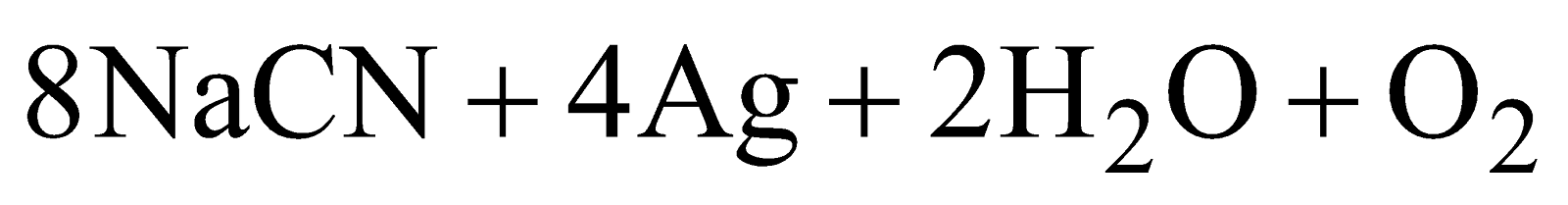

(b) 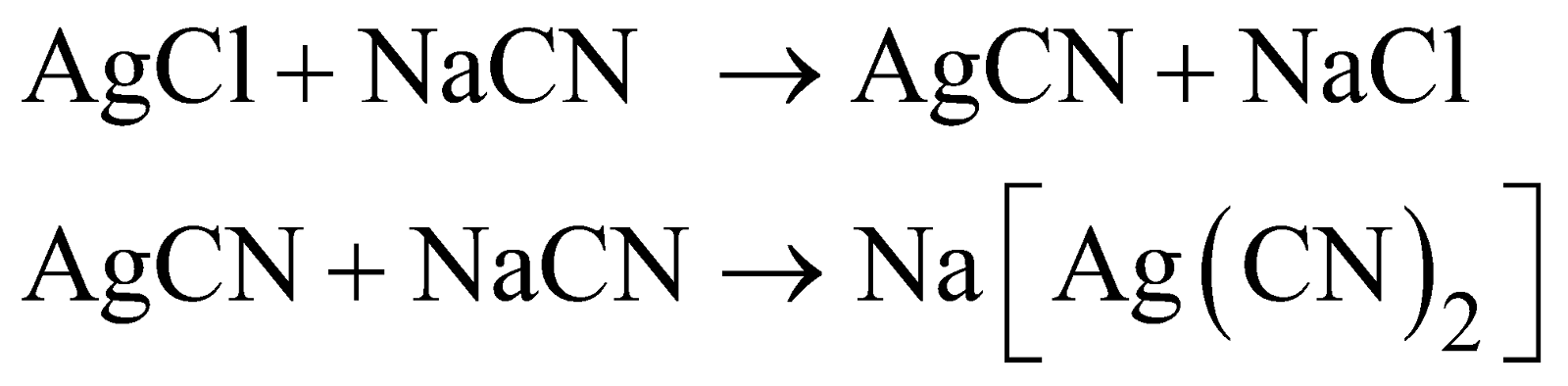
(c) 
Reversible reaction is prevented by oxidation of 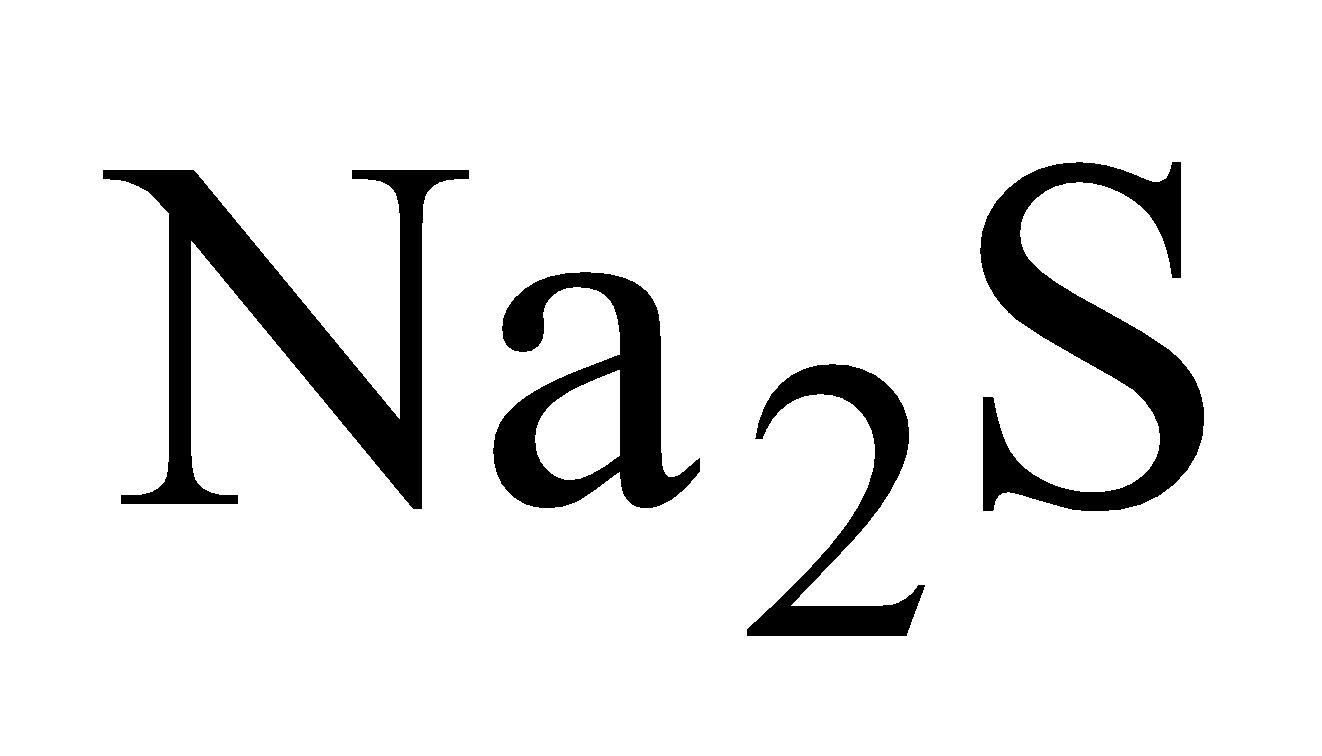 by air.
by air.
- Precipitation of silver - It is done with zinc.
- Refining
Electrolytic method
Anode - impure silver 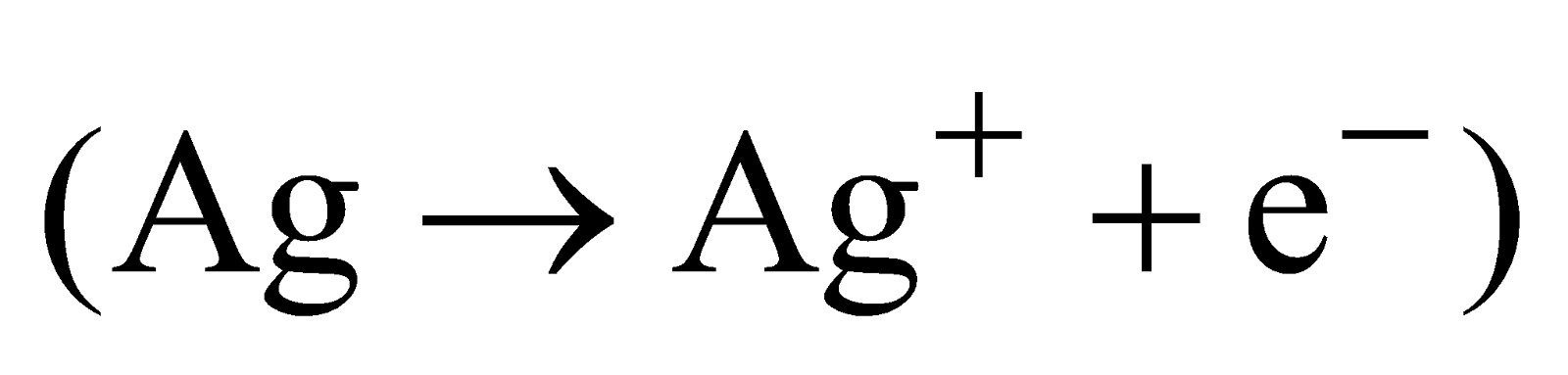
Cathode - pure silver 
Electrolyte - 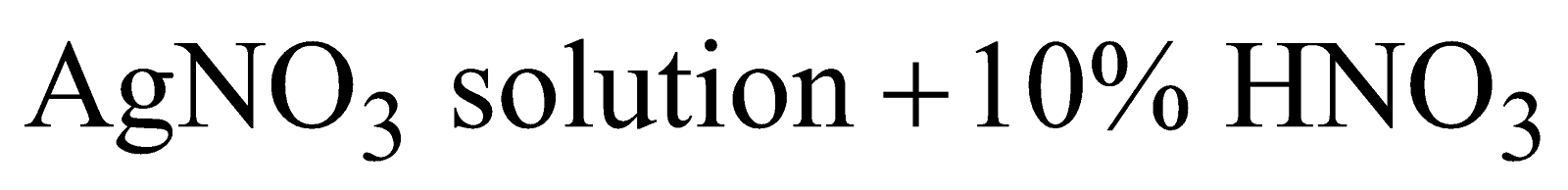
SILVER FROM ARGENTIFEROUS LEAD (DESILVERISATION OF LEAD)
Lead, extracted from galena (PbS) contains small amount of silver and is called argentiferous lead. Silver is recovered from it by-
- Parke’s process - Molten argentiferous lead is shaken with zinc when whole of silver passes into zinc. On cooling Ag- Zn alloy solidifies and being lighter floats over molten lead. It is separated, melted and distilled. Zinc distills over and silver is left behind. Success of the method depends upon the fact that-
(a) Silver is more soluble in molten zinc and
(b) Molten Zn and lead are immiscible
- Pattison’s process - (When silver is less than 1.0%).The lead-silver mixture containing 2.6%. Silver melts at lower temperature than pure lead. When molten argentiferous lead is allowed to cool pure lead solidifies first and removed. The silver content of the mixture is allowed to raise to 2.6%.The silver is then recovered by cupellation.
PROPERTIES
- It is a noble metal not attacked by atmospheric oxygen. The surface is tarnished due to formation of Ag2S due to H2S present in air.
- Dissolves in dilute and concentrated nitric acid
- Dissolves in alkali cyanide
- Dissolves in conc. sulphuric acid (not in dil. sulphuric acid)
USES
For making ornaments (80%Ag+20%Cu), electroplating, preparation of mirrors.
Fineness of Silver - It is the amount of silver present in 1000 parts of silver alloy. 925 fine silver means an alloy of 92.5% silver and 7.5%copper.
COMPOUNDS OF SILVER
SILVER NITRATE OR LUNAR CAUSTIC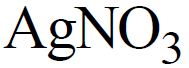
PREPARATION
By the action of dilute nitric acid on silver.
PROPERTIES
- It is colourless crystalline solid, soluble in water. It leaves black deposit when rubbed on the skin due to formation of finely divided silver.
- Action of heat
- Tollen’s reagent - The ammoniacal silver nitrate solution is known as Tollen’s reagent.
- Reaction with aqueous solution of certain compounds
USES
For silvering mirror ,electroplating, in medicines, for the preparation of silver halides used in photography. Particularly AgBr which is most sensitive to light.
GOLD (Au)
OCCURRENCE
It occurs free as Reef gold, Vein gold or auriferous quartz. Some improtant ores are
- Claverite
,
- Sylvanite
- Auriferous pyrites. These are sulphide ores of Cu, Ag, lead which contain gold.
EXTRACTION
By cyanide or Mac Arther Forest cyanide process
CONCENTRATION
Sulphides and tellurides are concentrated by froth floatation process.
Sulphides and tellurides are concentrated by froth floatation process.
ROASTING
The concentrated ore is roasted to remove oxidisable impurities of Te, As and S.
Formation of complex- NaCN solution is sprayed over the crushed ore and the gold with air, forming complex ion in solution.
The gold is then recovered as a solid by reduction.
PARTING
Removal of impurities of Ag and Cu from gold is known as parting.Impure gold is boiled with conc. when Ag and Cu dissolve and Au remains unaffected .
.
PURIFICATION
By electrolytic method using gold chloride 2.5-6.0% and conc. HCl.·
Plattner chlorine extraction process (From auriferous pyrites)
The moistened auriferous pyrites is saturated with chlorine, leached with water then treated with which precipitates gold.
Auriferous pyrites (moistened)
The moistened auriferous pyrites is saturated with chlorine, leached with water then treated with which precipitates gold.
Auriferous pyrites (moistened)
Impurities of Ag and Cu are removed by parting (as above).
PROPERTIES
- Pure gold is soft, hardened by Ag or Cu.
Fineness of gold - It is expressed in terms of carats. Pure gold is 24 carats. 22 carats mean it contains 22 parts by weight of gold and 2 parts by weight of other metals generally copper.
- It is very inert and not attacked by oxygen, water and acids.
- It is attacked by aqua regia
- It is attacked by chlorine.
- Auric chloride forms red crystals. Soluble in water and decomposed on heating.
ZINC (Zn)
OCCURRENCE
It is not found free in nature.The principal ores are -
- Zinc blende (sphalerite) ZnS
- Zincite or Red zinc oxide ZnO
- Franklinite
- Calamine or Zinc spar
- Willemite
PREPARATION
EXTRACTION
It is extracted by reduction process from ZnS (Zinc blende).
It is extracted by reduction process from ZnS (Zinc blende).
CONCENTRATION
The ore is concentrated by froth flotation process.
The ore is concentrated by froth flotation process.
ROASTING
The concentrated Zinc blende is roasted in a current of air.
The concentrated Zinc blende is roasted in a current of air.
(is utilised for the manufacturing of H2SO4)
If calamine ore is used, it is calcined.
REDUCTION
The ZnO is reduced by mixing with carbon and heating in fire clay retort.
PURIFICATION
Zinc so obtained contains the impurities of Fe, Pb, Cd, As or Sb. It is purified by
- Distillation or
- Electrolytic method
Anode impure : 
Cathode pure :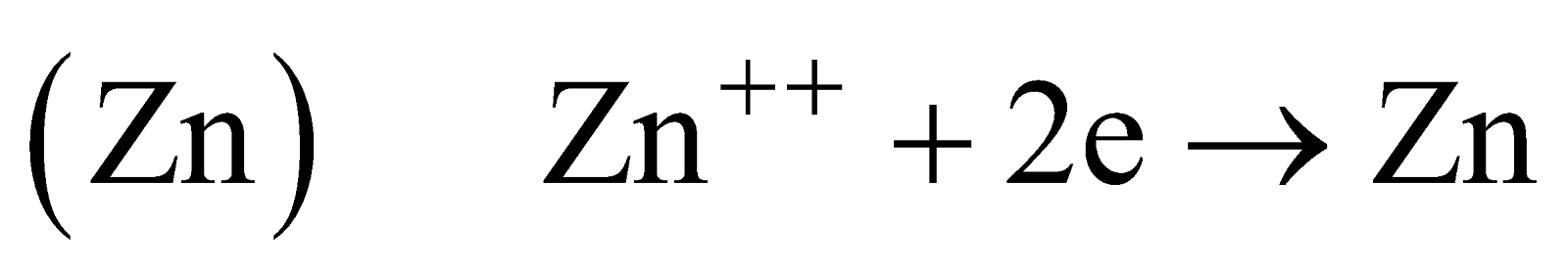
Electrolyte : Acidic solution of 
Liquation - Molten Zn is allowed to flow down on sloping hearth when non fusible impurities are left behind.
Electrolytic method – Pure 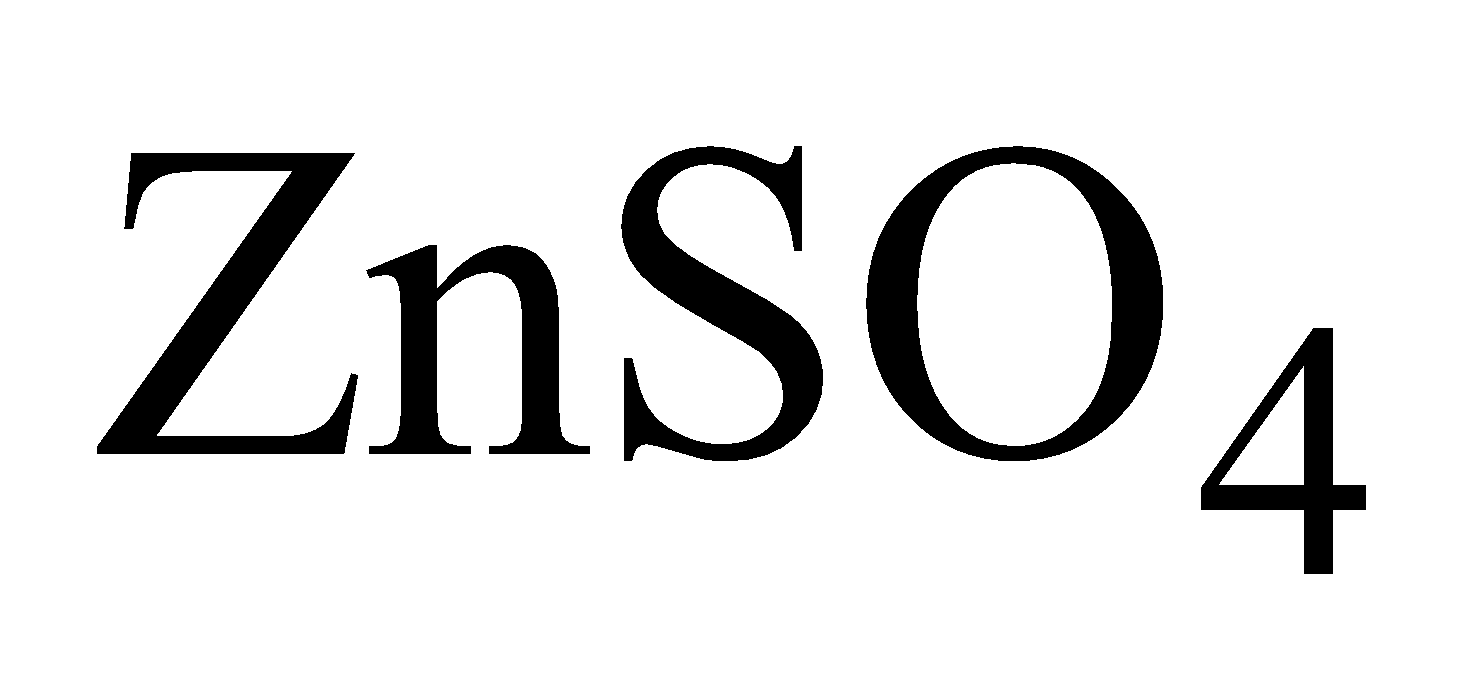 is electrolysed when Zn is deposited on aluminium cathode. It is scraped off and melted to obtain 99.95% pure metal.
is electrolysed when Zn is deposited on aluminium cathode. It is scraped off and melted to obtain 99.95% pure metal.
Zinc dust - It is prepared by atomising molten zinc with blast of air.
Granulated Zinc - It is prepared by pouring molten Zinc into cold water.
PHYSICAL PROPERTIES
- It is bluish white metal, stable in air.
- In moist air a protective covering of basic zinc carbonate is formed at its surface
- Action of heat - When heated to 500ºC it catches fire with bluish white flames forming ZnO which is very light and called philosopher's wool.
CHEMICAL PROPERTIES
- Action with acids
- Displacement reactions
- With non metals
- It is powerful reducing in nature.
USES
Galvanising, sherardizing, in Parke’s process for desilverisation of lead , for extraction of Ag and Au (Cyanide process). Zinc compounds are used in paints, filling rubber etc.
COMPOUNDS OF ZINC
ZINC OXIDE, PHILOSOPHER'S WOOL, ZINC WHITE OR CHINESE WHITE, ZnO
PREPARATION
PROPERTIES
- It is white powder becomes yellow on heating but again white on cooling.
- It sublimes at 673K.
- With alkali - It forms zincate.
- Reduction
- Dissolves in acids to form corresponding salts.
USES
As a white paint , in medicines, glaze in ceramics and filler in rubber industry.
WHITE VITRIOL
PREPARATION
By the action of dil. on Zinc metal, ZnO or
PROPERTIES
- Colourless, crystalline compound, highly soluble in water.
- Action of heat
USES
It is used to prepare lithophone 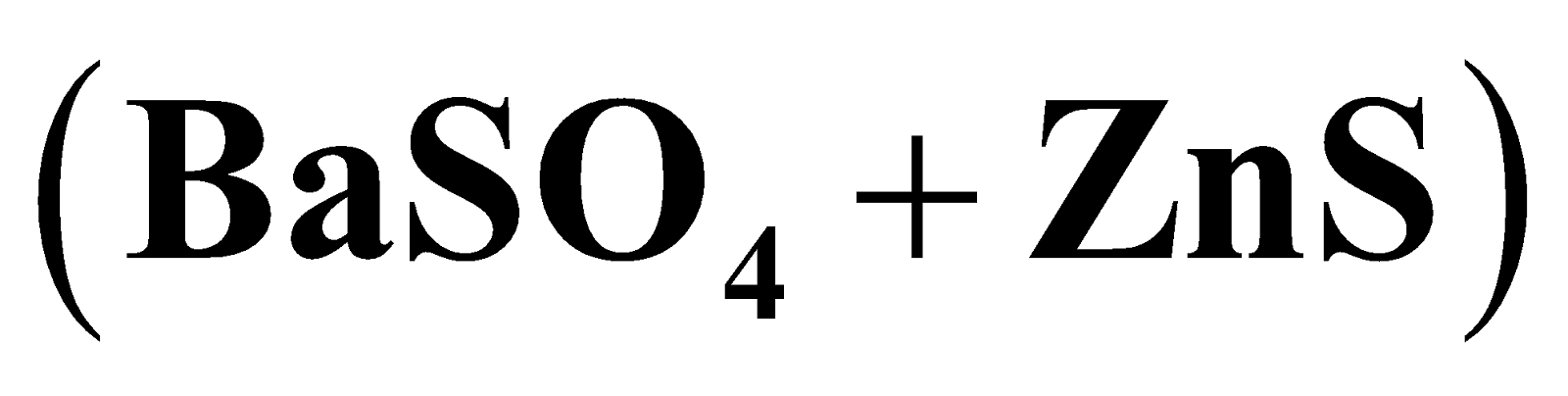 white pigment , galvanising iron and steel, as mordant in calico printing, in medicine as eye lotion.
white pigment , galvanising iron and steel, as mordant in calico printing, in medicine as eye lotion.
ZINC(II) CHLORIDE
PREPARATION
It is prepared by the action of dilute HCl on Zn, ZnO or ZnCO3
PROPERTIES
It is very deliquescent, soluble in water and organic solvents.
Anhydrous zinc chloride
USES
As timber preservative, flux in soldering, preparation of vulcanised paper and fibre.
MERCURY (Hg)
OCCURRENCE
It occurs in free state as small quantities. Its chief ore is -
- Cinnabar HgS
- Tiemannite
- Calomel Hg2Cl2
EXTRACTION
- Concentration - Cinnabar ore is concentrated by froth floatation process.
- Roasting - Roasting is carried out in a shaft furnace when mercury is obtained by auto reduction.
Roasting may be carried out with iron scrap or quicklime.
- Purification - It contains the impurities of Zn, Pb, Sn or Bi. Some of these impurities get oxidised in air and form a black scum on the surface. Finally it is purified by distillation in vacuum.
PROPERTIES
- Physical properties - It is silvery white liquid also known as Quick silver or live silver. It is the heaviest liquid known. Sp. gr. 13.59 at.
- Action of air - It is not attacked by air either dry or moist at ordinary temperature.
- It forms mercuric oxide at.
- Dilute acids have no action on mercury except dil..
- With concentrated acids
- Deadening of Hg - On Shaking vigorously alone or with fats or sugar it changes to grey powder. This is called deadening of mercury.
- Tailing of mercury - In presence of ozone it loses its meniscus which is known as tailing of mercury.
- Amalgams - The alloys of mercury with metals excerpts (Fe and Pt) are commonly known as amalgams.
- Ammonium amalgam - Sodium amalgam when placed in conc. solution of
, there is swelling and butter like mass is formed which is ammonium amalgam.
- Mercury tree - When small amount of Hg is poured into solution
. (Ag-Hg) is formed which grows like a tree and called mercury tree.
USES
In thermometers , barometers, electric cells etc.
COMPOUNDS OF MERCURY
MERCURIC OXIDES HgO
PREPARATION
- By heating mercury in air or O2
- By heating mercuric nitrate
- From mercuric chloride by the action of NaOH
Red HgO and yellow HgO differ in their particle size.
On heating yellow form changes to red.
USES
It is used as pigment in oil paints and as mild antiseptic in ointments.
MERCUROUS CHLORIDE OR CALOMEL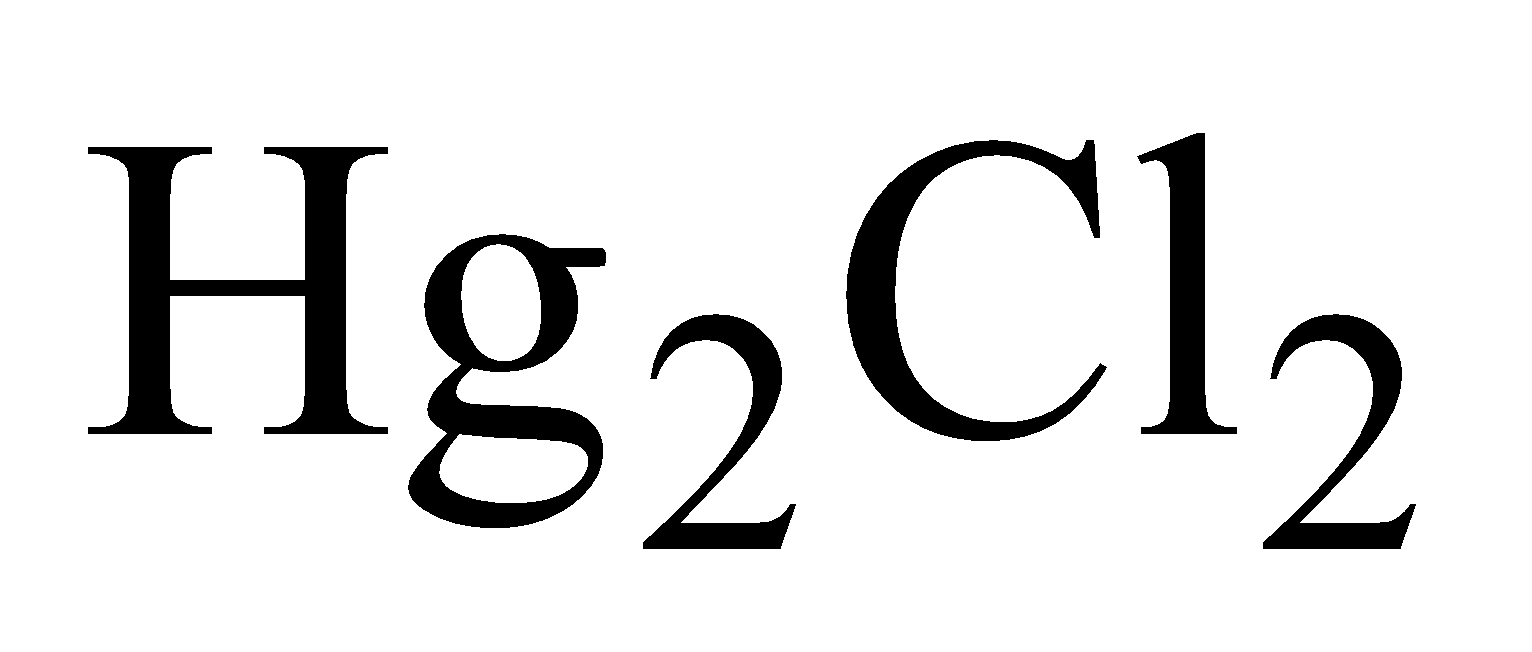
PREPARATION
- From mercurous nitrate
- From mercuric chloride by action of mercury
PROPERTIES
- It is insoluble in water purified by sublimation.
- Action of NH3 - It becomes black with ammonia.
- Action of heat - It is decomposed.
USES
In ceramics for golden colour and as calomel electrode.
MERCURIC CHLORIDE OR CORROSIVE SUBLIMATE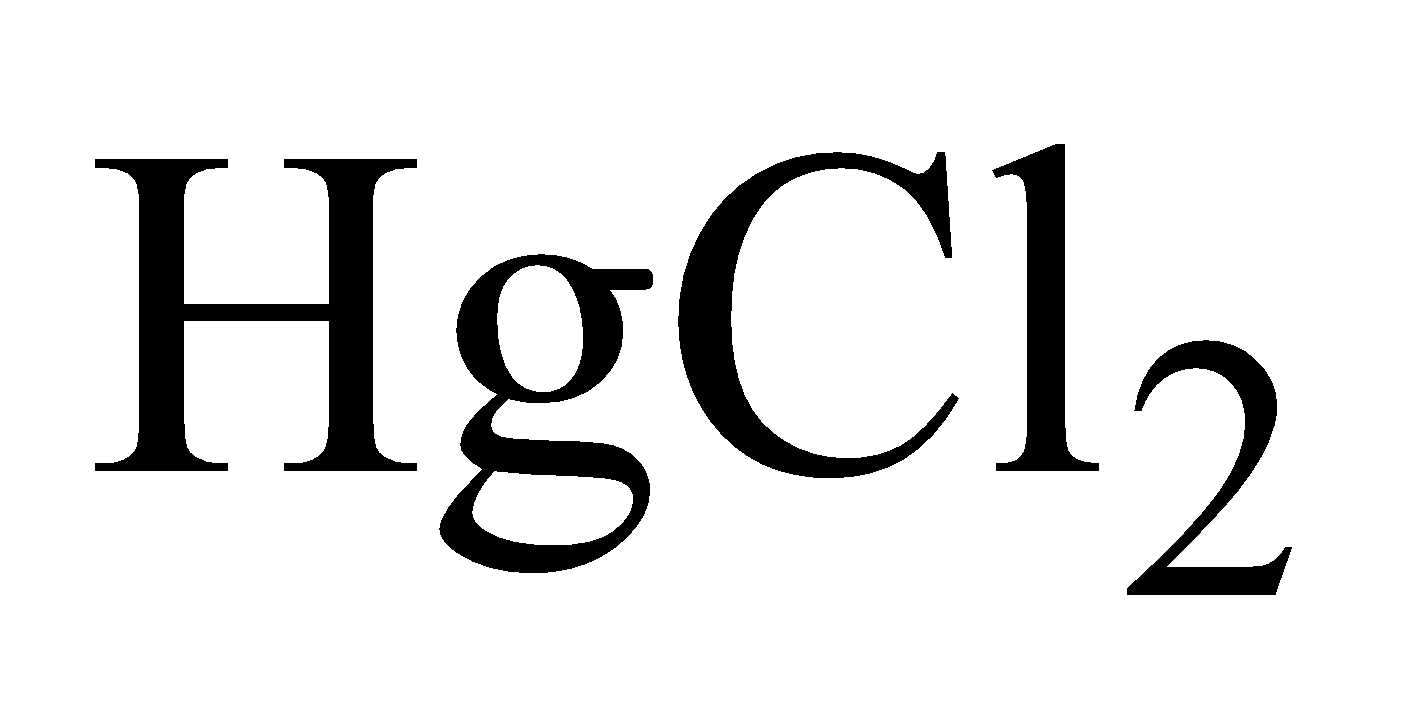
PREPARATION
By heating mercury in a current of chlorine.
MANUFACTURE
By heating mercuric sulphate with equal quantity of sodium chloride.
PROPERTIES
- Colourless, crystalline substance, covalent in nature and gives 5-8% solution in water.
- With SnCl2 - It is reduced to mercury
- With KI - It gives scarlet precipitate soluble in excess of KI.
- With NaOH - It gives HgO.
Nessler’s reagent - An alkaline solution of is known as Nessler’s reagent. It is used for the identification of ammonia and ammonia salts.
Brown (iodide of Millon’s base)
MERCURIC IODIDE
PREPARATION
By adding KI solution to any mercuric salt solution.
By adding KI solution to any mercuric salt solution.
PROPERTIES
Scarlet
Scarlet
It is soluble in excess of KI forming complex ion. Its alkaline solution is Nessler’s reagent as shown above.
MERCURIC SULPHATE
PREPARATION
By treating Hg with conc.
By treating Hg with conc.
PROPERTIES
It is white opaque mass, decomposes on heating to gives Hg (I) Sulphate.
It is white opaque mass, decomposes on heating to gives Hg (I) Sulphate.
COMPOUNDS OF MANGANESE
POTASSIUM PERMANGANATE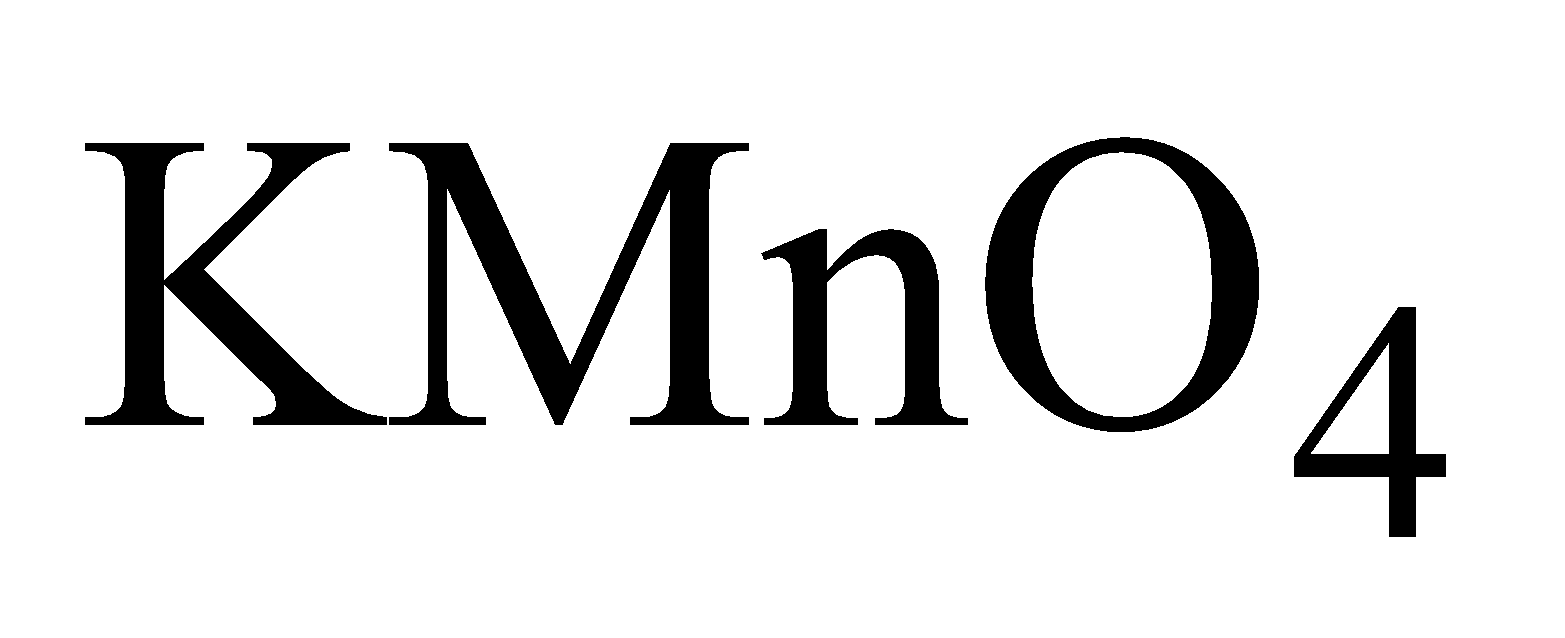
PREPARATION
On large scale it is prepared from  pyrolusite. The steps involved are as follows
pyrolusite. The steps involved are as follows
- Preparation of potassium manganate
- By fusing manganese dioxides with
.
- Oxidation of
To 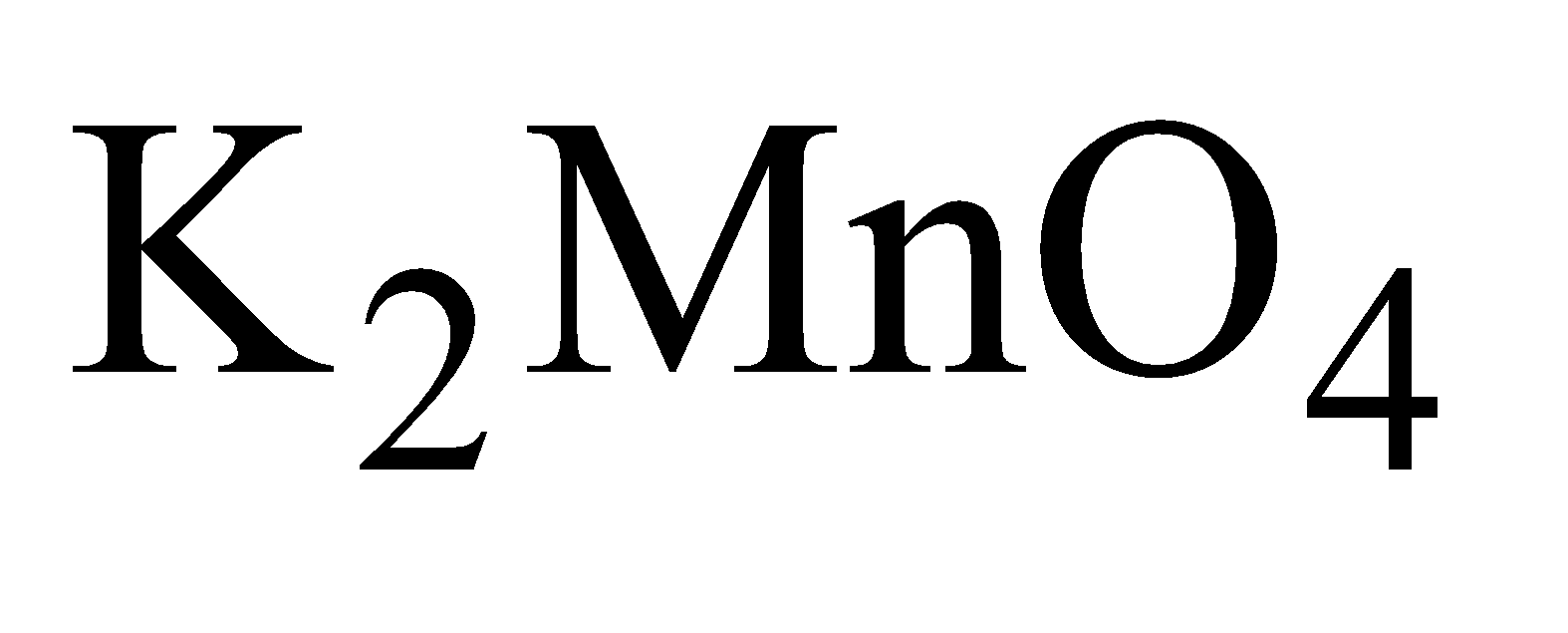 solution is either added
solution is either added
- H2SO4 or
(The last oxidation is known as STADELER ‘s process)
- Electrolytic oxidation - Nowadays electrolytic oxidation is prefered. The manganate solution is electrolysed between iron electrodes separated by diaphragm.
At anode 
At cathode 
PHYSICAL PROPERTIES
It is dark purple solid, soluble in water giving purple solution. Its melting point is 523 K.
It is dark purple solid, soluble in water giving purple solution. Its melting point is 523 K.
CHEMICAL PROPERTIES
- Action of heat -
- Oxidising nature - It is strong oxidising agent , both in alkali as well as in acidic medium and also in neutral.
In acidic medium
In alkaline medium
In neutral medium
In alkaline medium it oxides potassium iodide to potassium iodate and nitro toluene to nitro benzoic acid.
- Action of hydrogen - It burns on heating in a current of.
- Equivalent weight of
in different medium
- Equivalent weight in acid medium
- Equivalent weight in alkaline medium
- Equivalent weight in neutral medium
(See ionic equations above)
USES
As oxidising agent, disinfectant, 1% alkaline solution of KMnO4 is used to test unsaturation in organic compounds under the name of Baeyer’s reagent. It is used for the volumetric estimation of Fe++ salts,oxalic acid etc.
COMPOUNDS OF CHROMIUM
POTASSIUM DICHROMATE 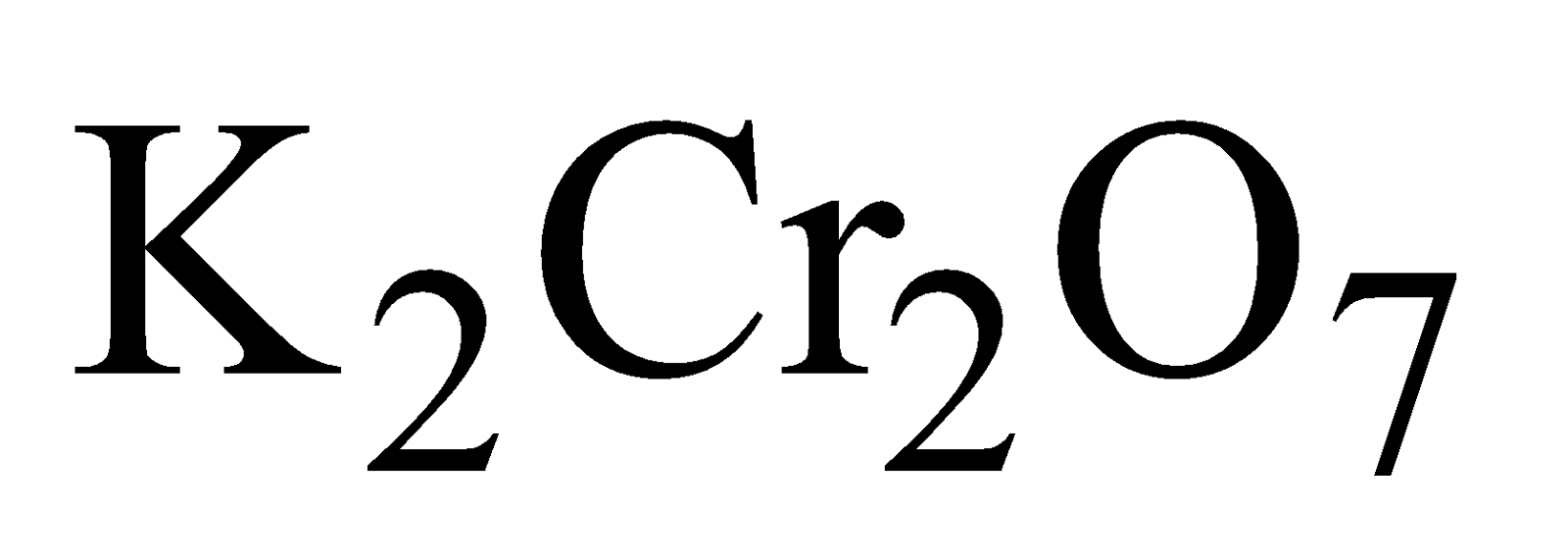
PREPARATION
It is manufactured from chromite ore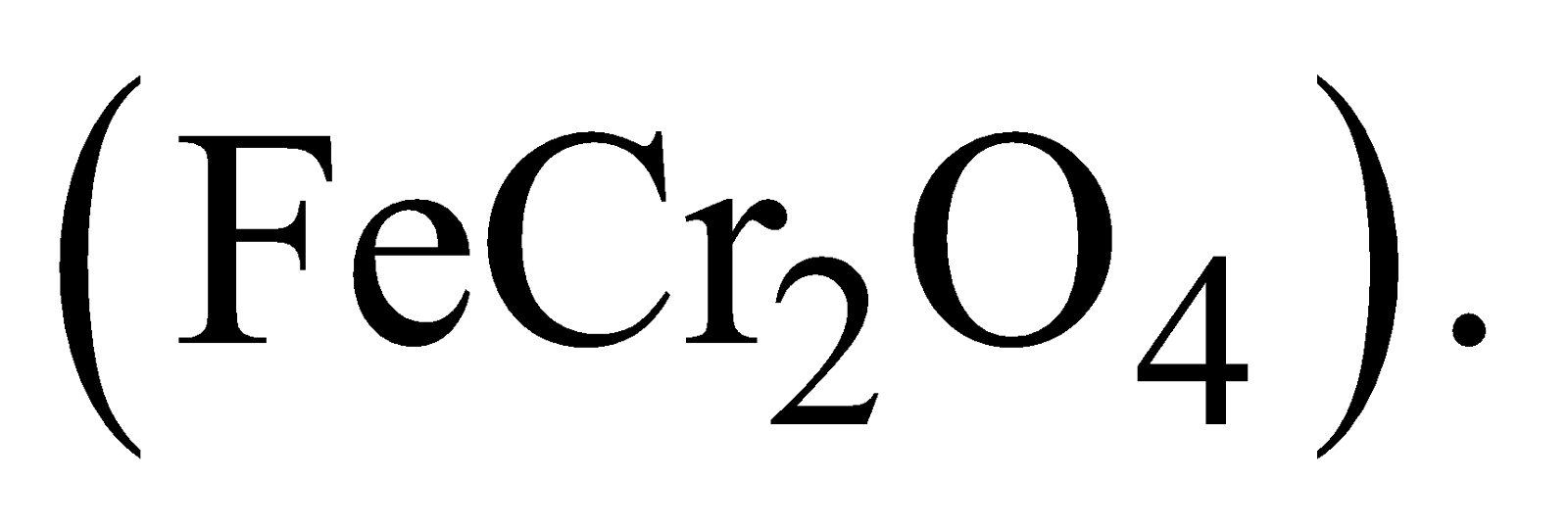 The steps involved are -
The steps involved are -
- Preparation of sodium dichromate - Finely powdered chromite is mixed with soda ash and quick lime and roasted in reverberatory furnace or rotary furnace in excess of air.
Chromite can be fused with molten alkali in presence of air.
The solution is filtered and acidified with dil. H2SO4 when sodium dichromate is obtained.
- Conversion of sodium dichromate to potassium dichromate.
Hot concentrated solution of Potassium dichromate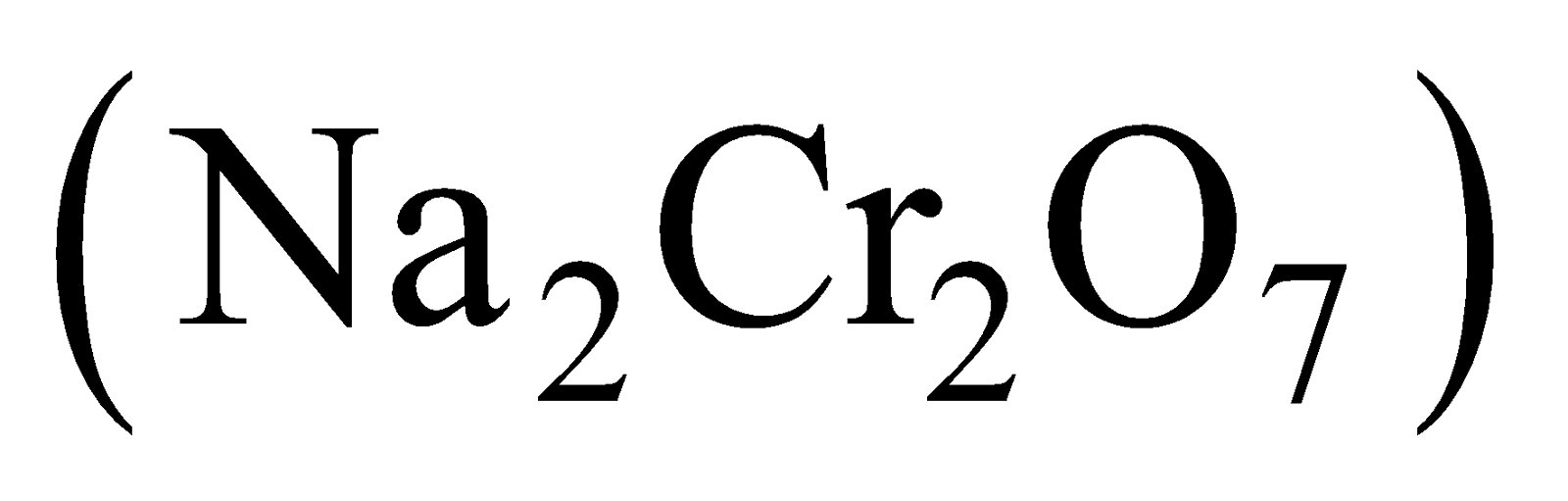 is less soluble and separates out on crystallisation.
is less soluble and separates out on crystallisation.
PROPERTIES
It is garnet red prismatic (orange) crystalline compound having melting point 398°C. Soluble in water.
CHEMICAL PROPERTIES
- Action of heat
- Action of cold
- Action of alkali
- Oxidising nature - It is powerful oxidising in nature.
- Formation of chromyl chloride - When a chloride is heated with potassium dichromate and conc. orange red vapour of chromyl chloride are formed.
- With lead salts it gives insoluble chromate salt.
USES
- In chrome tanning
- In dyeing-calico printing
- In photography
- Chromic acid
used as cleaning agent,
- In preparation of compounds such as
etc.
STRUCTURE
It consists of two tetrahedra with common oxygen atom
Dichromate ion
Structure of chromate ion : It has tetrahedral structure
At pH about 4 dichromate ion ( ) and chromate ion (
) and chromate ion (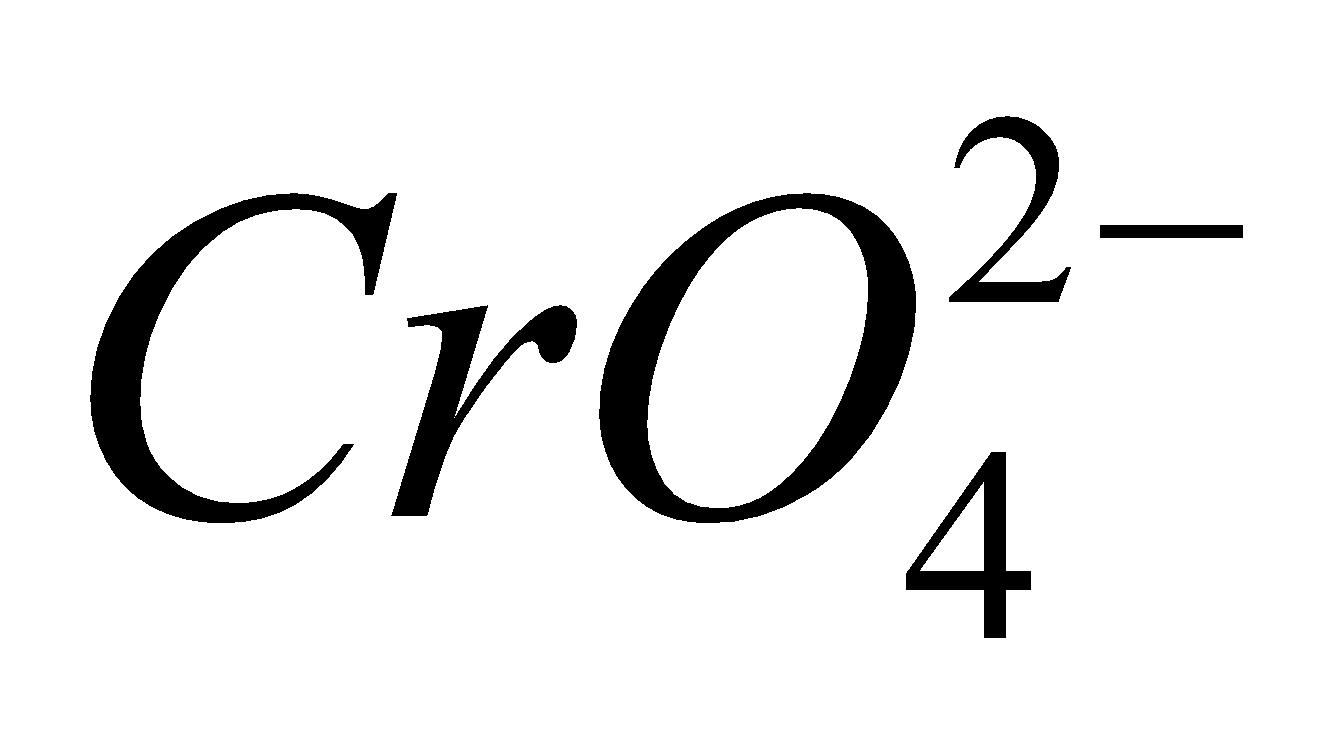 ) exist in equilibrium. These are interconvertible.
) exist in equilibrium. These are interconvertible.
INNER TRANSITION ELEMENTS
The elements in which the filling of atomic orbitals by electrons take place in f subshells, two levels inside the outer subshell, are known as inner transition elements. Thus these elements form a series within the transition series. They are also known as f- block elements since the differentiating electron enters the f -subshell.
CLASSIFICATION OF F-BLOCK ELEMENTS
They have been classified into two series.
- 4f-series (first inner transition series) - The differentiating electron enters in 4 f orbitals. The elements belonging to this series are also known as Lanthanides or Lanthanones.
- 5f -series (second inner transition series) -The differentiating electron enters in 5 f orbitals. The elements belonging to this series are also known as Actinides or Actinones.
For the sake of symmetry of the periodic table they have been placed outside the periodic table.
LANTHANIDES
The fifteen elements from lanthanum (At. no. 57) to lutetium (At. no. 71) are known as lanthanides or rare earths (because they were obtained as earths (oxides) from relatively rare minerals).
PROPERTIES
ELECTRONIC CONFIGURATION
The general electronic configuration of these elements is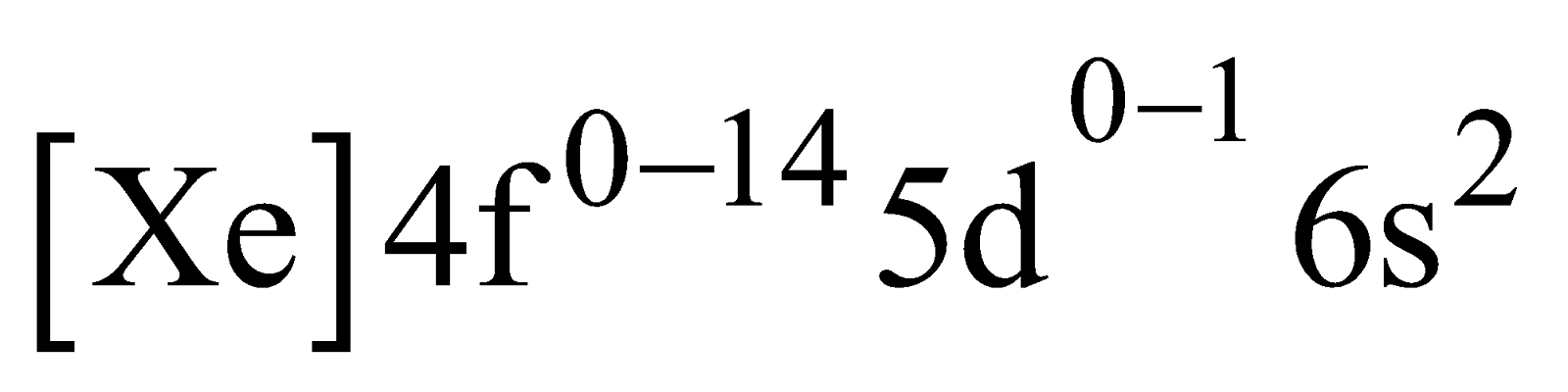
The lanthanum electronic configuration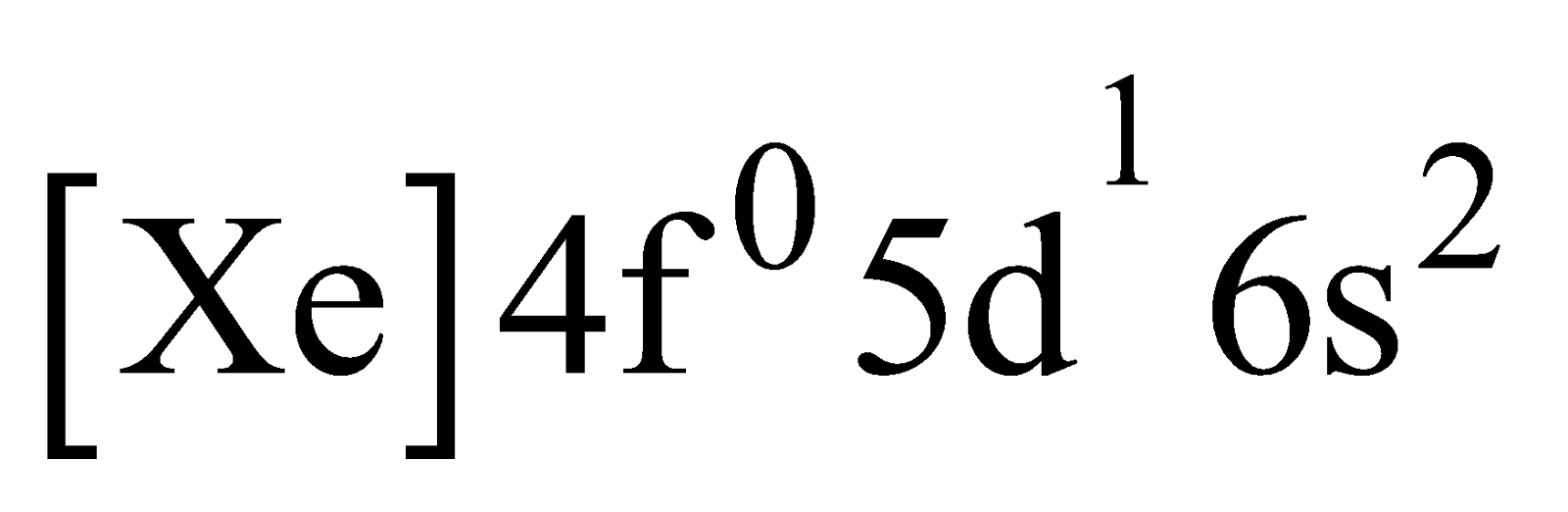 and lutetium electronic configuration
and lutetium electronic configuration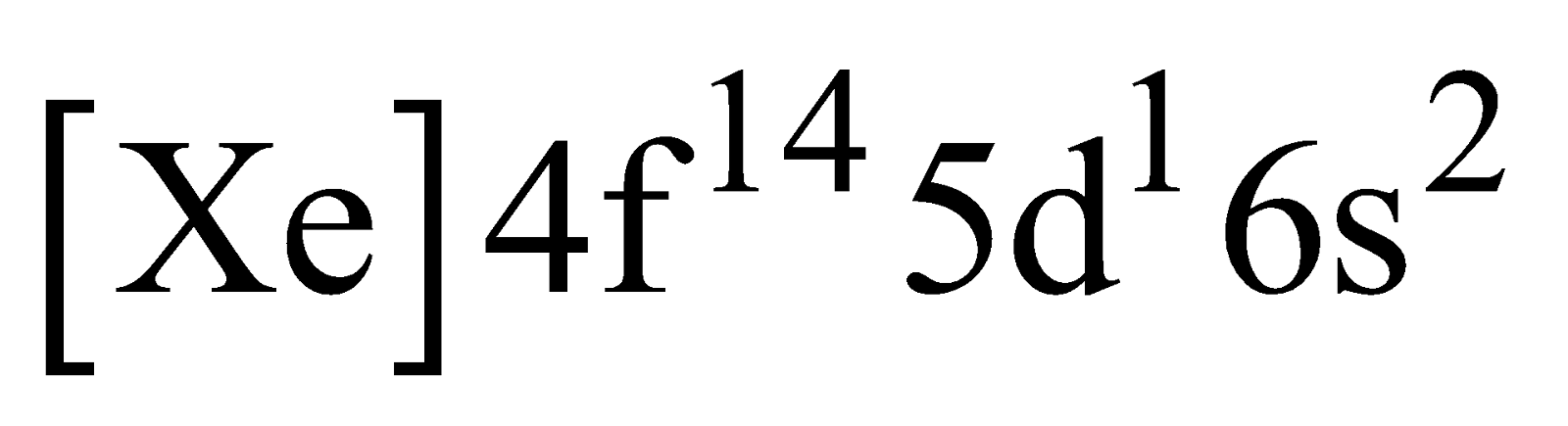 , have no partially filled 4 f orbital in their ground state, are considered as lanthanides due to their properties close to these elements.
, have no partially filled 4 f orbital in their ground state, are considered as lanthanides due to their properties close to these elements.
OXIDATION STATE
The common oxidation state of lanthanides is +3 but some elements also exhibit +2 and +4 oxidation states in which they leave behind stable ions eg.
An aqueous solution of is a good oxidising agent. The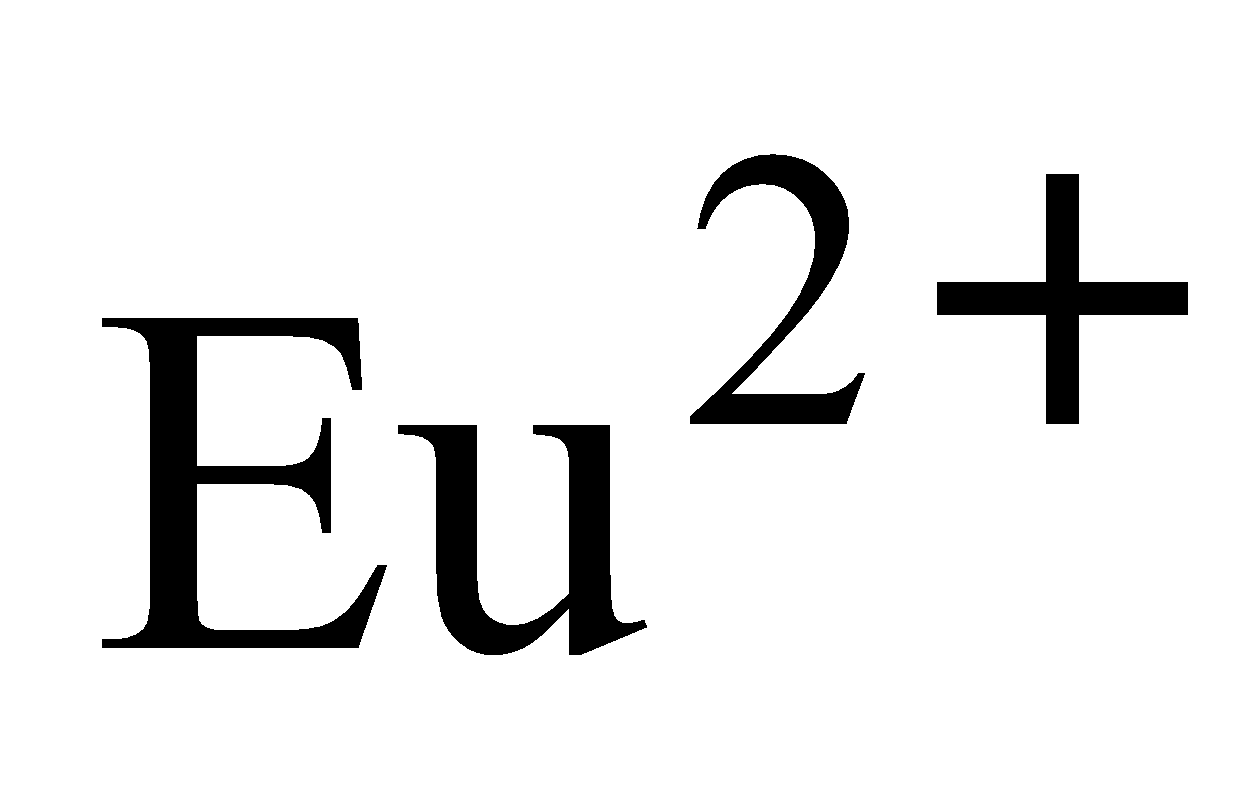 and
and 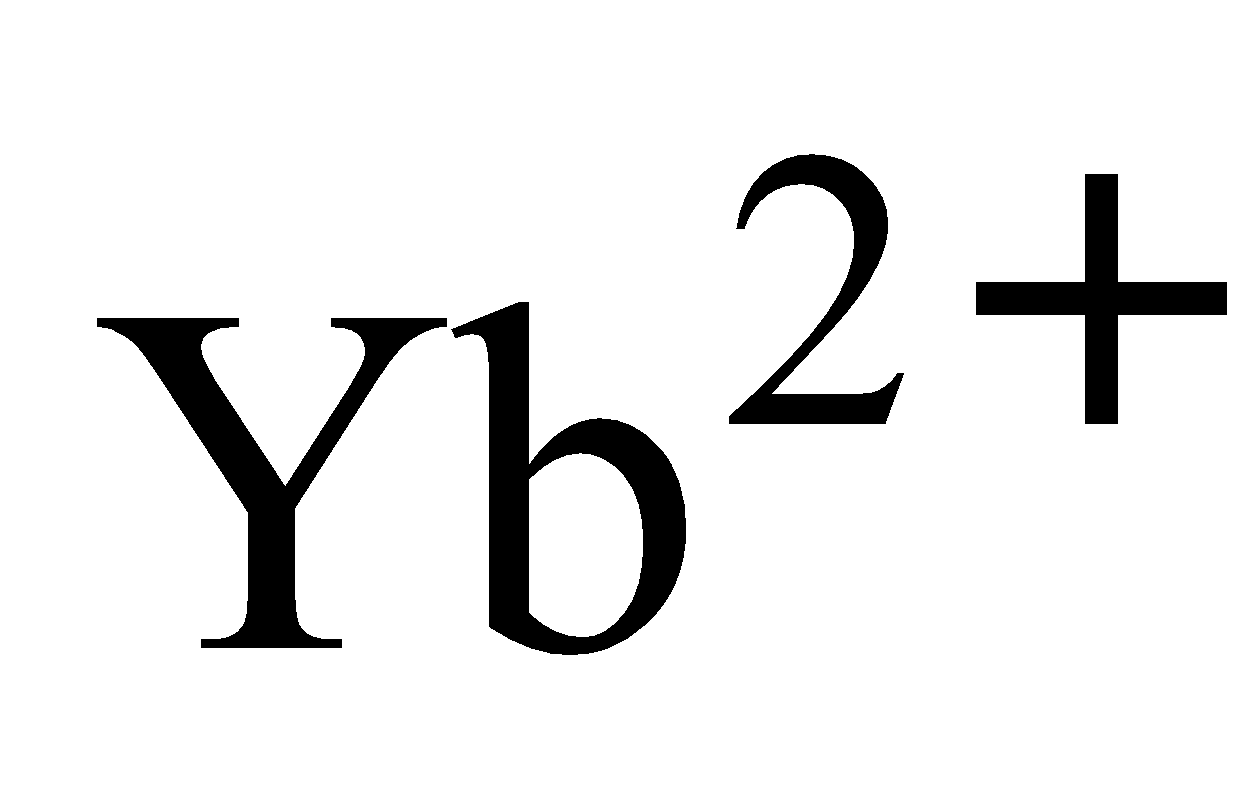 can exist in aqueous solution and are good reducing agents. But there are exceptions also e.g.
can exist in aqueous solution and are good reducing agents. But there are exceptions also e.g.
MAGNETIC PROPERTIES
Magnetic properties have spin and orbit contributions (Contrast “spin only”of transition metals). Hence magnetic momentums are given by the formula.
where L = Orbital quantum number, S = Spin quantum number
All lanthanide ions with the exception of  are paramagnetic in nature. The trend in magnetic moment is shown by graph.
are paramagnetic in nature. The trend in magnetic moment is shown by graph.
LANTHANIDE CONTRACTION
There is a steady decrease in the radii as the atomic number of the lanthanide elements increases. For every additional proton added in nucleus the corresponding electron goes to subshell. The shape of f -orbitals is very much diffused and they have poor shielding effect. The effective nuclear charge increases which causes the contraction in the size of electron charge cloud. This contraction in size is quite regular and known as Lanthanide contraction.
Consequences of lanthanide contraction
- Covalent character of cations increase.
- Electronegativity - The electronegativity of trivalent ions increase slightly.
- Basicity - There is decrease in basic strength of oxides and hydroxides.
- Eo value - There is small increase in standard electrode potential values.
COLOUR
The species containing unpaired electrons are coloured and so is the case with lanthanide ions. The f-f transitions are possible due to absorption of light from the visible region.
MELTING AND BOILING POINT
Lanthanides have high melting and boiling points but there is no regular trend.
DENSITY
Lanthanides have densities varying from. But there is no definite trend for these values.
ELECTRONEGATIVITY
Electronegativity values of lanthanides are almost same as that of s-block elements. Lanthanides form ionic compounds.
Electronegativity values of lanthanides are almost same as that of s-block elements. Lanthanides form ionic compounds.
IONISATION ENERGIES
The ionisation energy values of lanthanides are not very high due to their large size and are comparable with those of alkaline earth metals.
The ionisation energy values of lanthanides are not very high due to their large size and are comparable with those of alkaline earth metals.
COMPLEX COMPOUND
Due to having large ionic size they have little tendency to form complexes.
REACTIVITY
Due to their low values of ionisation energies the lanthanides are very reactive.
ALLOYS
They form alloy especially with iron e.g. MISCH METAL rare earths
ACTINIDES
The fifteen elements from actinium (At. no. 89) to lawrencium (At. no. 103) are known as actinides and constitute the 5f. Series. From neptunium to onwards the elements are man made (artificially prepared) and also known as transuranium elements.
PROPERTIES
ELECTRONIC CONFIGURATION
The differentiating electron enters the 5f atomic orbital. Their general electronic configuration is
Since there is not much difference between 5f and 6d, it is difficult to predict whether the electron has entered 5f or 6d.
OXIDATION STATE
The common oxidation state is +3 but other oxidation states are also exhibited by actinides the maximum being +7.
MAGNETIC PROPERTIES
The magnetic moments of actinide ions are smaller than theoretical values.
It is hard to interpret due to large spin orbit coupling.
It is hard to interpret due to large spin orbit coupling.
ACTINIDE CONTRACTION
It is similar to lanthanide contraction due to poor shielding of electrons.
MELTING AND BOILING POINTS
They have high values for melting and boiling points but there is no regular trend.
DENSITY
The value of density vary from 7.0 gcm–3 to 19.84 gcm–3. Again there is no regular trend.
REDUCING CHARACTER
They are strong reducing agents as they have high values approximately 2.0 volts.
REACTIVITY
Actinides are very reactive in nature and combine with oxygen and halogens like lanthanides.
COLOURED IONS
Actinide ions are coloured due to the presence of unpaired electrons and transitions.
COMPLEX FORMATION
They have higher tendency to form complex compounds.










