ELECTROCHEMISTRY
ELECTROLYTES AND ELECTROLYSIS
A substance which decomposes as a result of the passage of electric current is called an electrolyte and phenomenon of decomposition by electricity is called electrolysis.
ELECTROLYTIC CELL
The apparatus used to carry out electrolysis is known as electrolytic cell. The main features of electrolytic cell are :
Feature
|
Cathode
|
Anode
|
Sign
|
Negative since attached to negative end of external battery
|
Positive since attached to positive end of external battery
|
Direction of movement of electrons
|
Into the cell
|
Out of the cell
|
Direction of movement of ions
|
Cations
|
Anions
|
Half-Reaction
|
Reduction
|
Oxidation
|
Oxidation : Loss of electrons is called oxidation.
Reduction : Gain of electrons is called reduction.
FARADAY'S LAWS OF ELECTROLYSIS
FIRST LAW
The amount of the substance deposited or liberated at an electrode is directly proportional to the quantity of electricity passed through an electrolyte
I = Current strength in amp., t = time in sec.,
Q = Quantity of charge (coulombs)
Z is a constant known as Electrochemical equivalent
When I = 1 amp., t = 1 sec. then Q = 1 coulomb, then W = Z.
Thus, Electrochemical equivalent is the amount of the substance deposited or liberated by 1 ampere current passing for 1 second (i.e. 1 coulomb, I × t = Q)
SECOND LAW
When the same quantity of electricity is passed through different electrolytes, the amounts of the products obtained at the electrodes are directly proportional to their chemical equivalents or equivalent weights. Thus
Hence, Electrochemical equivalent ∝ Equivalent wt.
ONE FARADAY
One Faraday is the quantity of charge carried by one mole of electrons
AMOUNT OF THE SUBSTANCE 'm' LIBERATED OR DEPOSITED AT AN ELECTRODE
COMPARISON OF ELECTROLYTIC AND METALLIC CONDUCTORS
Property
|
Metallic Conductor
|
Electrolytic Conductor
|
Conduction
|
Due to movement of electrons
|
Due to movement of ions
|
Transfer of matter
|
No transfer of matter
|
There is transfer of matter
|
Chemical property
|
No change in chemical property
|
There is chemical change
|
Resistance
|
Resistance is offered by atomic Kernels
|
Resistance is offered by interionic attractions, viscosity of solvent
|
Temperature
|
Decreases with increase of temperature
|
Increases with increase of temperature
|
Magnitude
|
Generally high
|
Generally low
|
Solvation
|
No solvation
|
Ions are solvated
|
FACTORS AFFECTING ELECTROLYTIC CONDUCTION
Please refer to the properties discussed above viz. viscosity, temperature, solvation of ions etc.
OHM'S LAW
The potential difference across the conductor is directly proportional to the current flowing through it.
Potential difference ∝ Current
where R is a constant and known as resistance of the conductor
Law is applicable to metallic as well as electrolytic conductors. R is expressed in Ohms also designated as  .
.
RESISTANCE (R)
It offers obstruction to the passage of electric current. It is directly proportional to the length (l) and inversely proportional to the area of cross section (a) of the conductor.
where r is a constant, called resistivity or specific resistance.
when l = 1 cm and a = 1 cm2 then  = R
= R
SPECIFIC RESISTANCE (ρ)
The resistance offered by 1 cm3 of the conductor is known as specific resistance.
Units of 
CONDUCTANCE (C)
It is ease of flow of electric current through the conductor and reciprocal of resistance R.
C =  units ohm–1, mho or
units ohm–1, mho or  –1
–1
SPECIFIC CONDUCTIVITY 𝛋 (KAPPA)
It is the reciprocal of specific resistance.
Hence specific conductivity 𝛋 (Kappa) = Conductance × cell constant
Units = Ohm–1 cm–1 =  –1 cm–1 = S cm–1 (
–1 cm–1 = S cm–1 ( –1 = S, Siemens)
–1 = S, Siemens)
EFFECT OF DILUTION ON CONDUCTANCE
The number of current carrying particles or ions per ml decrease on dilution and specific conductivity, being the conductance of one centimetre cube of solution, decreases with dilution.
CELL CONSTANT AND ITS DETERMINATION
The quantity  is known as cell constant. Its direct measurement is very difficult. It is measured by using standard solution of KCl whose conductivity is known at different concentrations and temperatures.
is known as cell constant. Its direct measurement is very difficult. It is measured by using standard solution of KCl whose conductivity is known at different concentrations and temperatures.
Hence cell constant = 
EQUIVALENT CONDUCTIVITY 
The conductivity of all the ions produced when 1 gram equivalent of an electrolyte is dissolved in V ml of solution is known as equivalent conductivity.
or
The conductance of a solution containing 1 gm. equivalent of electrolyte placed between two large electrodes one centimetre apart.
RELATION BETWEEN EQUIVALENT CONDUCTIVITY AND SPECIFIC CONDUCTIVITY (
AND SPECIFIC CONDUCTIVITY ( )
)
where V is the volume in cm3 or ml containing 1 gev. of the electrolyte.
UNITS OF EQUIVALENT CONDUCTIVITY
Ohm–1 cm2 eq–1 or  –1 cm2 eq–1 or S cm2 eq–1
–1 cm2 eq–1 or S cm2 eq–1
EFFECT OF DILUTION ON EQUIVALENT CONDUCTIVITY
Since the degree of dissociation of the electrolyte increases with dilution, the equivalent conductivity also increases. The increase is more in case of weak electrolytes than strong electrolytes.
The equivalent conductivity increases and specific conductivity decreases with dilution.
IMPORTANCE OF EQUIVALENT CONDUCTIVITY
It helps to compare the conductivity of different electrolytes, since solutions of different electrolytes having 1 gram equivalent each in the same volume will have the same total charge of electricity. One mole of NaCl yields ions carrying 2 faradays of electricity and one mole of Na2SO4 yields ions carrying 4 faradays of electricity. But 1 gram equivalent of each will produce ions carrying 2 faradays of electricity.
Equivalent wt. of Na2SO4 = 
Hence conductivity of different electrolytes can only be compared if their solutions have equivalent concentrations.
MOLAR CONDUCTIVITY ( )
)
The conductivity of all the ions produced when 1 mole of an electrolyte is dissolved in V ml of solution is known as molar conductivity.
or
The conductance of a solution containing one gram-mole of electrolyte placed between two large electrodes one centimeter apart.
RELATION BETWEEN MOLAR CONDUCTIVITY ( ) & SPECIFIC CONDUCTIVITY (
) & SPECIFIC CONDUCTIVITY ( )
)
where V is the volume in cm3 or ml containing 1 mole of the electrolyte.
UNITS OF MOLAR CONDUCTIVITY ( )
)
Ohm–1 cm2 mol–1 or  –1 cm2 mol–1 or S cm2 mol–1
–1 cm2 mol–1 or S cm2 mol–1
EFFECT OF DILUTION ON MOLAR CONDUCTIVITY
Since the degree of dissociation of electrolyte increases with dilution,  also increases but less in case of strong electrolytes and more in case of weak electrolytes.
also increases but less in case of strong electrolytes and more in case of weak electrolytes.
FACTORS AFFECTING THE MOLAR CONDUCTIVITY
- Nature of electrolyte : The strong electrolytes like KNO3, KCl, NH4NO3, HCl, H2SO4, NaOH, KOH etc are completely ionised in aqueous solution and have high values of
. The weak electrolytes are ionised to lesser extent in aqueous solution and have lower values of
.
- Concentration of the solution : The concentrated solutions of strong electrolytes have significant inter-ionic attractions, which reduce the speed of ions and lower the value of Lm. The dilution decreases such attractions and increases the value of
. The limiting value
(
the molar conductivity at zero concentration or at infinite dilution) can be obtained by extrapolating the graph.
In case of weak electrolytes, the degree of ionisation increases which increases the value of  . The limiting value cannot be obtained by extrapolating the graph. The limiting value,
. The limiting value cannot be obtained by extrapolating the graph. The limiting value, , for weak electrolytes is obtained by Kohlrausch law.
, for weak electrolytes is obtained by Kohlrausch law.
- Temperature : The increase of temperature decreases interionic attractions, solvation of ions, viscosity and increases kinetic energy of ions and their speed. Thus
increases with temperature.
- Viscosity of solvent : The higher the value of viscosity the lower is the value of
.
- Dielectric constant of solvent : The higher the value of dielectric constant of solvent, the more is the value of
. The former decreases interionic attractions.
DEBYE-HUCKEL ONSAGER EQUATION
Relation between molar conductivity  at a particular concentration and molar conductivity
at a particular concentration and molar conductivity  at infinite dilution is given by
at infinite dilution is given by
It depends upon nature of solvent and temperature.
DEGREE OF DISSOCIATION ( )
)
For weak electrolytes
KOHLRAUSCH'S LAW
At infinite dilution the molar conductivity of an electrolyte is the sum of the ionic conductivities of the cations and anions. e.g. for AxBy.
It is important to note that the source of cations (A+) and anions (B–) may be electrolyte itself or any other electrolyte.
IONIC MOBILITY
The distance travelled by an ion per second under a potential gradient of 1 volt per cm is known as ionic mobility.
Ionic Mobility U = 
POTENTIAL GRADIENT
Potential difference applied at the electrodes divided by the distance between the electrodes is known as Potential gradient.
GALVANIC CELL, ELECTROCHEMICAL CELL OR VOLTAIC CELL
A device which converts chemical energy into electrical energy is called Galvanic, Electrochemical or voltaic cell.
General representation of an electrochemical cell
Cathode
|
Anode
| |
Sign
|
Positive due to consumption of electrons
|
Negative due to release of electrons
|
Reaction
|
Reduction
|
Oxidation
|
Movement of electrons
|
Into the cell
|
Out of cell
|
Other features of the electrochemical cell are
- There is no evolution of heat
- The solutions remain neutral on both side
- The reaction and flow of electrons stops after some time.
DANIELL CELL
An electrochemical cell of Zinc and copper metals is known as Daniell Cell. It is represented as
Zn (s) | Zn++ (aq.) | | Cu2+ (aqs.) | Cu (s)
LHS oxidation : 
RHS reduction : 
Overall reaction : 
By convention cathode is represented on the RHS and anode on the LHS. Two vertical lines represent the salt bridge.
FUNCTION OF SALT BRIDGE
- Completes the circuit and allows the flow of current
- It maintain the electrical neutrality on both sides.
Salt-bridge generally contains solution of strong electrolyte such as KNO3, KCl etc. KCl is preferred because the transport numbers of K+ and Cl– are almost same.
ELECTRODE POTENTIAL
When an electrode is in contact with the solution of its own ions in a half cell, it has a tendency to lose or gain electrons which is known as electrode potential. It is expressed in volts.
OXIDATION POTENTIAL
The tendency to lose electrons in above case is known as Oxidation potential.
Oxidation potential of a half-cell is inversely proportional to the concentration of ions in the solution.
REDUCTION POTENTIAL
The tendency to gain electrons in above case is known as reduction potential.
It is not possible to determine the absolute value of electrode potential. For this a reference electrode is required. The electrode potential is only the difference of potentials between two electrodes that we can measure by combining them to give a complete cell.
STANDARD REDUCTION POTENTIAL ( )
)
According to latest convention the electrode potential is always represented as reduction potential. If its value is negative, it means electrode has oxidation potential. The standard conditions are 1 molal solution, 298K temperature and 1atm. pressure. According to IUPAC convention, the reduction potential alone be called as the electrode potential unless it is specifically mentioned.
REFERENCE ELECTRODE
The following electrodes are used as reference electrodes for determining the standard reduction potentials.
STANDARD HYDROGEN ELECTRODE (SHE)
Standard hydrogen electrode (SHE) also known as Normal Hydrogen Electrode (NHE), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The wire is sealed into a glass tube, placed in a beaker containing 1M HCl. The hydrogen gas at 1 atm pressure is bubbled through the solution at 298K. Half cell is Pt, H2(1 atm)/H+ (1M)
In SHE, at the surface of platinum, either of the following reactions can take place
The electrode potential of SHE has been fixed as zero.
All other single electrode potentials are referred to as potentials on hydrogen scale.
Drawbacks of SHE
- It is difficult to maintain H2 at 1 atm. pressure.
- It is difficult to maintain H+ ion concentration at 1M
- The platinum electrode is easily poisoned by traces of impurities.
Hence calomel and silver chloride electrodes are conveniently used as reference electrodes.
CALOMEL ELECTRODE (Hg, Hg2Cl2, KCl)
Half cell is Hg, Hg2Cl2(s) KCl(solution)
Oxidation :
Reduction :
Overall reaction :
EoRed = 0.2422 V
SILVER-SILVER SALT ELECTRODE
Half cell is Ag, AgCl(s), Cl– (KCl or HCl)
Oxidation :
Reduction :
ELECTROMOTIVE FORCE (EMF) OF A CELL
It is the difference between the electrode potentials of two half-cells and cause of flow of current from electrode at higher potential to electrode at lower potential. It is also the measure of free energy change. Standard EMF of a cell
ELECTROCHEMICAL SERIES
It is the arrangement of electrodes in the increasing order of their Standard Reduction potential . 
APPLICATIONS OF ELECTROCHEMICAL SERIES
- The lower the value of E°, the greater the tendency to form cation
- Replacement (or evolution) of H2 from hydro acids by metals.
Metals placed below hydrogen in E.C.S. can not replace hydrogen from dil. acids but metals placed above hydrogen can replace hydrogen from dil. acids.
Ca + H2SO4 ⟶ CaSO4 + H2 ↑
possible
Cu + H2SO4 ⟶ CuSO4 + H2 ↑
not possible
- Metals placed above hydrogen evolve H2 with H2O or steam, but metals placed below hydrogen cannot.
- Oxides of metals placed above hydrogen are not reduced by H2 but oxides of iron and metals placed below iron are reduced by H2
SnO, PbO, Fe3O4, CuO are reduced with H2
CaO, MgO, K2O are not reduced with H2
- Oxides of Ag and metals below Ag are decomposed
- Reducing character decreases down the series.
- Reactivity decreases down the series.
- Feasibility of redox reaction : A redox reaction can occur if the species losing the electrons lie above that which gains the electrons.
- Determination of emf : Emf is the difference of reduction potentials of two half cells.
Eemf = ERHS – ELHS
If the value of e.m.f. is positive the reaction can take place spontaneously, otherwise not.
FUNCTIONING OF ELECTROCHEMICAL CELL
With the passage of time the electrode potential of the cathode decreases and that of anode increases the difference becomes zero, the driving force of emf becomes zero and reaction stops.
TYPES OF ELECTRODES AND HALF-CELLS
GAS ELECTRODES OR GAS ION HALF CELLS
Half-cell Reaction
Oxidation
Reduction
METAL-METAL ION ELECTRODE OR HALF-CELL
A metal rod dipped in the solution of its own ions
Zn++(aq) / Zn : 
Ag+(aq) / Ag :
Cu++(aq) / Cu :
METAL-METAL INSOLUBLE SALT-SALT ANION
Cl–(aq) / AgCl / Ag: 
Br–(aq) / AgBr / Ag : 
Cl–(aq) / Hg2Cl2(s) / Hg (Pt) : 
OH–(aq) / Cu(OH)2(s) / Cu : 
REDOX ELECTRODES HALF CELLS
An inert metal such as platinum wire dipped in a solution of ions of the same metal in different oxidation states
Fe3+(aq), Fe2+(aq) / Pt :
Sn4+(aq), Sn2+(aq) / Pt: 
Cr2O72–(aq), Cr3+(aq), H+(aq) / Pt :
MnO–4 (aq); Mn2+(aq), H+(aq) / Pt :
NERNST EQUATION FOR ELECTRODE POTENTIAL
The relationship between the concentration of ions and electrode potential is given by Nernst equation.
If we write the electrode reaction, in general, as 
The potential of electrode is given by
or 
NERNST EQUATION FOR CELL POTENTIAL
Consider a general cell reaction involving n electrons.
Concentration of solids and liquids is taken as unity, concentration of ions Mol L–1 and concentration of gases as partial pressures in Atmosphere.
NERNST EQUATION AND EQUILIBRIUM CONSTANT (Kc)
When Ecell drops to zero the concentration of ions will be equilibrium concentrations. We have for general cell reaction
RELATION BETWEEN ELECTRICAL ENERGY AND GIBBS FREE ENERGY ( G)
G)
If n is the number of electrons liberated (or taken up) in a particular cell reaction, then n faradays (nF) of electricity will be generated in the complete reaction. If E is the EMF of the cell, then
Electrical energy supplied by the cell = nFE
According to Gibbs and Helmholtz, the decrease of free energy (– G) of the reaction occuring in the cell is equal to electrical energy
G) of the reaction occuring in the cell is equal to electrical energy
Hence, – G = nFE
G = nFE
The standard free energy and are related as
are related as
RELATIONSHIP BETWEEN FREE ENERGY CHANGE AND EQUILIBRIUM CONSTANT :
ΔGo = -2.303RTlog KC
CONCENTRATION CELLS
ELECTRODE CONCENTRATION CELLS
Two electrodes of different concentrations are dipped in the same solution of electrolyte e.g.
ELECTROLYTE CONCENTRATION CELLS
Electrodes are the same but electrolyte solutions have different concentrations e.g.
APPLICATIONS OF THE CONCENTRATION CELLS
- Determination of valency
- Determination of solubility of sparingly soluble salts.
- Determination of transition point.
OVERVOLTAGE
The difference between the voltage at which a gas is actually evolved and theoretical value at which it ought to have been evolved during electrolysis is known as overvoltage.
REVERSIBLE CELL
When the cell reaction can be stopped or reversed by applying an emf exactly equal to or infinitesimally greater than that of the cell, it is called reversible cell.
EXAMPLES OF REVERSIBLE ELECTRODES
METAL - METAL ION ELECTRODE
Metal rod dipped into a solution of its own ions.
The negative electrode (electrode reaction involving oxidation) increases M+ ions in solution
The positive electrode (electrode reaction involving reduction) decreases M+ ions in solution.
Thus, electrode is reversible with respect to M+ ions
GAS ELECTRODES
- Hydrogen electrode : Pt, H2(g), H+
Reaction
The electrode is reversible with respect to H+ ions
- Chlorine electrode : Pt, Cl2(g), Cl–
Reaction
The electrode is reversible with respect to Cl– ion.
METAL - METAL SALT ION ELECTRODE
- Calomel Electrode : Hg, Hg2Cl2(s) ; KCl(solution)
Oxidation :
Oxidation decreases concentration of chloride ions.
Reduction :
Reduction increases the concentration of chloride ions
The electrode is reversible with respect to Cl– ions.
- Silver-Silver Chloride Electrode : Ag, AgCl(s), Cl–(KCl or HCl)
Oxidation :
Oxidation decreases the concentration of Cl– ions.
Reduction :
Reduction increases the concentration of Cl–
OXIDATION - REDUCTION ELECTRODES
Such electrodes are set up by inserting unattackable metal (eg: Platinum) into a solution of ions in different oxidation states. The metal acquires a potential due to tendency of ions in one oxidation state to change into another stable oxidation state.
Electrode reaction :
IRREVERSIBLE CELL
When the cell reaction cannot be stopped or reversed it is called irreversible cell e.g. cell of Zn and Ag electrodes immersed in solution of H2SO4.
CRITERIA OF THE FORMATION OF PRODUCTS IN ELECTROLYSIS (PREFERENTIAL DISCHARGE THEORY)
Ions present in large excess conduct electricity and ions having lower discharge potentials are discharged at respective electrodes.
Electrolysis of aqueous NaOH
At cathode : 
At anode : 
Current is carried by Na+ and OH– ions.
Electrolysis of aqueous H2SO4
At cathode : 
At anode : 
Electrolysis of aqueous NaCl
At cathode : 
At anode : 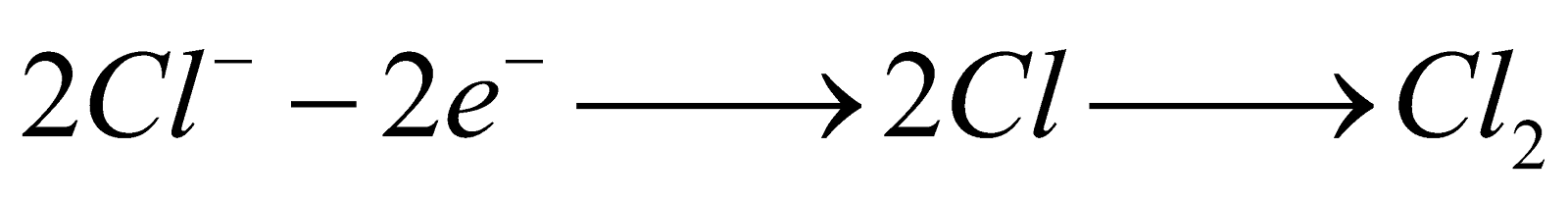
Although the oxidation potential of OH– is more than Cl– yet Cl2 is formed at anode due to overvoltage.
Electrolysis of aqueous CuSO4 using Pt electrodes
At cathode 
At anode 
Electrolysis of CuSO4 solution using copper electrodes
At Cathode 
At Anode 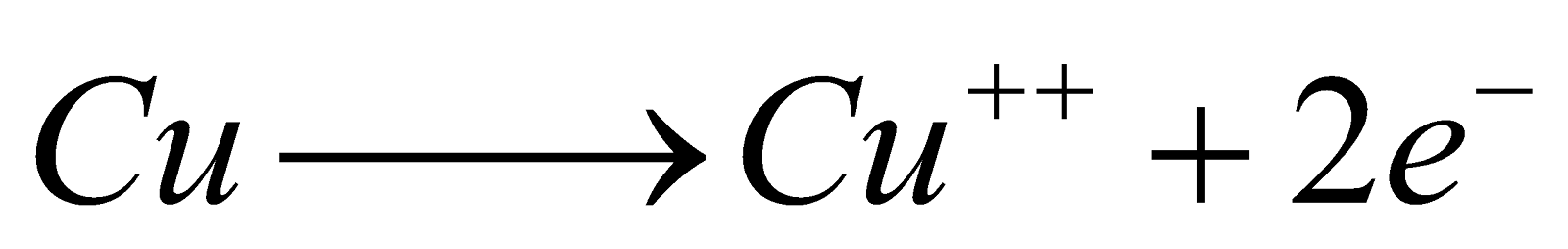
Oxidation potential 
FACTORS GOVERNING THE DISCHARGE POTENTIAL
- Position in the electrochemical series
- Concentration of ions in the solution
- Nature of electrodes
Discharge potential of some ions
TRANSPORT NUMBER
The fraction of the total current carried by each ion is called its transport number.
The amount of electricity carried by a particular ion speed of particular ion.
Transport number of the cation nc =  =
= 
Transport number of anion na = 
Uc = speed of cation and Ua = speed of anion.
Further na + nc = 1
SOME COMMERCIAL CELLS AND THEIR TYPES
They are broadly classified into two groups.
PRIMARY CELLS
They cannot be recharged and used again.
DRY CELL OR LECLANCHE CELL
Anode : Zinc Container
Cathode : graphite rod surrounded by MnO2 powder
Electrolyte : paste of NH4Cl + ZnCl2
Cathode Reaction : MnO2 +  +
+  e– MnO(OH) + NH3
e– MnO(OH) + NH3
Anode Reaction : 
Net Reaction : Zn2+ + 2NH3 [Zn(NH3)2]2+
[Zn(NH3)2]2+
Cell Potential : 1.25V to 1.5V
MERCURY CELL
Anode : Zn-Hg amalgam
Cathode : paste of (HgO + C)
Electrolyte : moist paste of KOH-ZnO
Cathode Reaction :
Anode Reaction : 
Net Reaction : 
Cell Potential : 1.30V
SECONDARY CELLS
It can be recharged and can be used again and again.
LEAD STORAGE BATTERY
Anode : Spongy lead
Cathode : grid of lead packed with PbO2
Electrolyte : 38% H2SO4 by mass
Anode Reaction : 
Cathode Reaction :
Net Reaction :
When recharged the cell reactions are reversed.
NICKEL-CADMIUM STORAGE CELL
Anode : Cadmium
Cathode : metal grid containing NiO2
Electrolyte : KOH solution
Anode Reaction : 
Cathode Reaction : 
Net Reaction : 
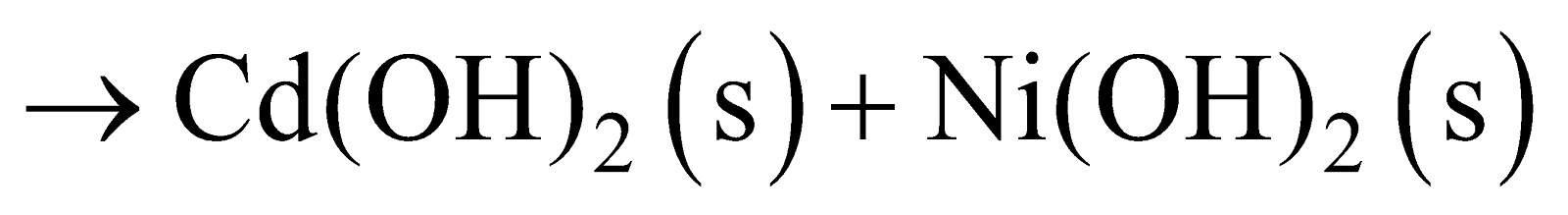
Cell Potential : 1.4 V
FUEL CELLS
They convert chemical energy into electrical energy and the reactants are continuously fed and products are removed.
HYDROGEN-OXYGEN FUEL CELL
Electrodes are made of porous graphite impregnated with catalyst (Pt, Ag or a metal oxide).
Electrolyte-aqueous solution of KOH or NaOH
Oxygen and hydrogen are continuously fed into the cell.
Oxidation (half-cell reaction) : 
Reduction (half-cell reaction) : 
Net Reaction :
EMF of the cell 1 volt.
HYDROCARBON-OXYGEN FUEL CELL
Based upon the combustion of hydrocarbons such as methane, ethane propane etc.
Oxidation (half cell reaction) : 
Reduction (half cell reaction) :
Net Reaction : C3H8 + 5O2  3CO2 + 4H2O
3CO2 + 4H2O
ADVANTAGES OF FUEL CELLS
- Pollution free
- High efficiency
THERMODYNAMIC EFFICIENCY OF FUEL CELLS
For H2–O2 fuel cells it is 95%.
CORROSION
Slow formation of undesirable compounds such as oxides, sulphides or carbonates at the surface of metals by reaction with moisture and other atmospheric gases is known as corrosion.
FACTORS AFFECTING THE CORROSION
- Reactivity of metals
- Presence of moisture and atmospheric gases like CO2, SO2 etc.
- Presence of impurities
- Strains in the metal
- Presence of electrolyte
RUSTING OF IRON - ELECTROCHEMICAL THEORY
An electrochemical cell known as corrosion cell is developed at the surface of iron.
Anode : Pure iron
Cathode : Impure Surface
Electrolyte : 





Anode Reaction :
Cathode Reaction :
Net Reaction : 
At surface : 
Net reaction at surface : 
PREVENTION OF CORROSION
- Barrier protection : By painting, coating, electroplating
- Sacrificial protection : By galvanization, sherardising
- Electrical protection
- Use of anti rust compounds.
CALCULATION OF POTENTIAL OF INTERMEDIATE REACTION
When two half-reactions having potential E1 and E2 which are combined to yield a third half reaction having potential E3. Then E3 is given by
Remember : The cell potentials are not thermodynamic functions and should not be added.










