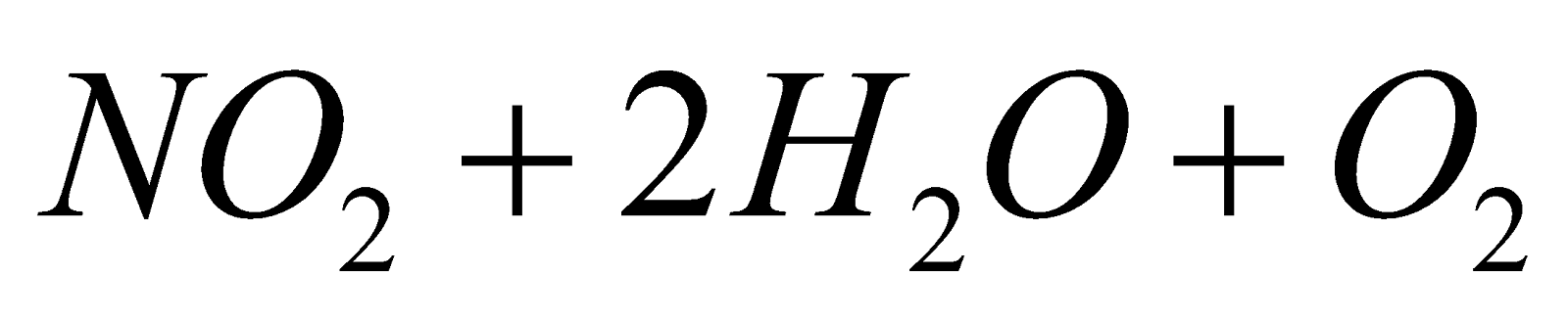PRINCIPLES RELATED TO PRACTICAL CHEMISTRY (PART-3)
DETECTION OF BASIC RADICALS - GROUP III
Members : Fe+++, Al+++, Cr+++
Group reagent : NH4OH in presence of NH4Cl (excess)
Precautions
- Before oxidation with HNO3, H2S must be completely removed otherwise it will be partly oxidised to H2SO4 which will precipitate the sulphates of Ba, Sr, etc.
- The soln is boiled with conc HNO3 to oxidise Fe++ ions to Fe+++ to ensure complete precipitation of iron as Fe(OH)3. Fe(OH)2 is not completely precipitated by NH4OH in presence of NH4Cl and will pass in the filtrate of IV gp
2FeCl2+ 8HNO3  2Fe(NO3)3 + 4HCl + 2NO2 + 2H2O
2Fe(NO3)3 + 4HCl + 2NO2 + 2H2O
- Mn is usually precipitated in gp IV but sometimes due to insufficient quantity of NH4Cl and presence of air bubbles in soln, it is precipitated with the radicals of Group III as manganic hydroxide MnO.OH
- The precipitation should be carried out in hot soln otherwise Cr may be incompletely precipitated due to the formation of violet soluble complex chromamine in presence of excess of NH4Cl. The complex may be decomposed by boiling.
- Large excess of NH4OH should be avoided because Al(OH)3 dissolves giving complex compound.
- Addition of NH4Cl is absolutely essential otherwise the basic radicals of subsequent gps ppt out as hydroxides in this gp
NH4Cl suppresses the ionisation of NH4OH and prevents the precipitation of higher group hydroxides in Group III.
Function of NH4Cl
- The hydroxides of Fe, Al and Cr have a strong tendency to pass into colloidal state. These are coagulated by NH4Cl which is a strong electrolyte
- The hydroxides of Al, Cr, and Zn are amphoteric. They supply negative ions in soln which form insoluble salts with other metallic ions (eg CrO2, Co(AlO2)3, Fe (ZnO2)3 etc. This entrapment of metallic ions by these hydroxides is known as “carrying down” . It is prevented by NH4Cl.
- The hydroxides of III group absorb other metal ions in the form of hydroxides eg [XFe(OH)3.YNi(OH)2] such hydroxide formation is prevented by NH4Cl separation.
Separation
By treating with Na2O2 or NaOH and Br2 water the hydroxides of Cr and Al dissolve as sodium chromate and aluminate respectively. While Fe(OH)3 and manganic hydroxide remain unaffected. MnO.OH is oxidised by PbO2 and HNO3 to permanganic acid HMnO4 which gives violet or pink colour. Fe(OH)3 dissolves in HCl forming FeCl3.
IRON (Fe+++)
Fe++ salts are easily oxidised when heated with HNO3
6FeSO4 + 3H2SO4 + 2HNO3  3Fe2(SO4)3 + 2NO + 4H2O
3Fe2(SO4)3 + 2NO + 4H2O
- NH4OH
FeCl3 + 3NH4OHFe(OH)3 ↓ + 3NH4Cl
Reddish brown
Fe(OH)3 + 3HCl  FeCl3 + 3H2O
FeCl3 + 3H2O
- K4[Fe(CN)6]
4FeCl3 + 3K4[Fe(CN)6]  Fe4[Fe(CN)6]3 ↓ + 12KCl
Fe4[Fe(CN)6]3 ↓ + 12KCl
Prussian Blue
FeCl3 + K4[Fe(CN)6]  KFe[Fe(CN)6] + 3KCl
KFe[Fe(CN)6] + 3KCl
K. ferric ferrocyanide
- NH4CNS or KCNS
FeCl3 + KCNS  Fe(CNS)Cl2 ↓ + KCl
Fe(CNS)Cl2 ↓ + KCl
Blood red colouration
others believe that complex ferric sulphocyanide is formed
2FeCl3 + 6NH4CNS  Fe[Fe(CNS)6] + 6NH4Cl
Fe[Fe(CNS)6] + 6NH4Cl
- Sodium acetate solution
FeCl3 + 3CH3COONa  3NaCl + Fe(CH3COO)3 ↓
3NaCl + Fe(CH3COO)3 ↓
reddish brown colouration
ALUMINIUM (Al+++)
- NH4OH
Al2(SO4)3 + 6NH4OH2Al(OH)3 ↓+ (NH4)2SO4
white gelatinous ppt
Al(OH)3 + 3HCl  AlCl3+ 3H2O
AlCl3+ 3H2O
Soluble
Al(OH)3 + NaOH  NaAlO2 + 2H2O
NaAlO2 + 2H2O
sod. aluminate (soluble)
- NH4Cl
NaAlO2 + NH4Cl + H2O  Al(OH)3 + NH3 + NaCl
Al(OH)3 + NH3 + NaCl
NaAlO2 + HCl + H2O  Al(OH)3 + H2O + NaCl
Al(OH)3 + H2O + NaCl
Al(OH)3 + 3HCl AlCl3 + 3H2O
AlCl3 + 3H2O
CHROMIUM (Cr+++)
- NH4OH
CrCl3 + 3NH4OH Cr(OH)3 + 3NH4Cl
Cr(OH)3 + 3NH4Cl
Bluish green gelatinous ppt
Cr(OH)3 + 6NH4OH  [Cr(NH3)6](OH)2 + 6H2O
[Cr(NH3)6](OH)2 + 6H2O
Violet pink soln
(chrome - ammine)
- NaOH and Br2 water
2Na2O2 + H2O 4NaOH + 2O
4NaOH + 2O
Br2 + NaOH NaBr + HBr + O
NaBr + HBr + O
2Cr(OH)3 + 4NaOH + 3O  2Na2CrO4 + 5H2O
2Na2CrO4 + 5H2O
Sod. chromate (yellow)
- Lead acetate
Na2CrO4 + (CH3COO)2Pb PbCrO4
PbCrO4  + 2CH3COONa
+ 2CH3COONa
Yellow
PbCrO4 + 4NaOH  Na2PbO2 + Na2CrO4 + 2H2O
Na2PbO2 + Na2CrO4 + 2H2O
Soluble
DETECTION OF BASIC RADICALS - GROUP IV
The ppt consists NiS, CoS, ZnS and MnS
ZINC (Zn++) AND MANGANESE (Mn++)
- Separation : ppt + dil HCl
ZnS and MnS dissolve as ZnCl2 and MnCl2 and NiS and CoS remain insoluble
ZnS + 2HCl  ZnCl2 + H2S
ZnCl2 + H2S
MnS + 2HCl  MnCl2 + H2S
MnCl2 + H2S
The soln is heated with NaOH
ZnCl2 + 2NaOH  Zn(OH)2 + 2NaCl ppt
Zn(OH)2 + 2NaCl ppt
MnCl2 + 2NaOH Mn(OH)2 + 2NaCl ppt
Mn(OH)2 + 2NaCl ppt
- Excess NaOH :
Zn(OH)2 + 2NaOH  Na2ZnO2 + 2H2O
Na2ZnO2 + 2H2O
Sod. Zincate soluble
Na2ZnO2 + H2S  ZnS + 2NaOH
ZnS + 2NaOH
White ppt
Na2ZnO2 + 2CH3COOH  (CH3COO)2Zn + 2NaOH
(CH3COO)2Zn + 2NaOH
2(CH3COO)2 Zn + K4[Fe(CN)6]  Zn2[Fe(CN)6] + 4CH3COOK
Zn2[Fe(CN)6] + 4CH3COOK
Bluish white
- Residue + Conc HNO3 +PbO2 or Pb3O4
Pink Coloured permanganic acid
Mn(OH)2 + 2HNO3 Mn(NO3)2 + 2H2O
Mn(NO3)2 + 2H2O
Pb3O4 + 4HNO3  2Pb(NO3)2 + PbO2 + 2H2O
2Pb(NO3)2 + PbO2 + 2H2O
5PbO2 + 2Mn(NO3)2 + 6HNO3 2KMnO4 + 5Pb(NO3)2
2KMnO4 + 5Pb(NO3)2
permanganic acid (pink)
permanganic acid (pink)
- With oxidising fusion mixture green mass of Na2MnO4
2KNO3 + 2NaOH + Mn(OH)2  2KNO2+ Na2MnO4 + H2O
2KNO2+ Na2MnO4 + H2O
sod. manganate (green)
NICKEL (Ni++) AND COBALT (Co++)
NiS and CoS soluble in aqua regia
3NiS + 3HNO3 + 6HCl  3NiCl2 + 2NO + 3S + 4H2O
3NiCl2 + 2NO + 3S + 4H2O
3CoS + 3HNO3 + 6HCl  3CoCl2 + 2NO + 3S + 4H2O
3CoCl2 + 2NO + 3S + 4H2O
- Method I
Nickel salt with DMG in ammoniacal medium gives red ppt. of NDMG
Cobaltous (Co++) salt with amm. thio cyanate gives blue colour due to
CoCl2+ 4NH4CNS  (NH4)2Co(CNS)4 + 2NH4Cl
(NH4)2Co(CNS)4 + 2NH4Cl
Amm. Cobalto thiocyanate blue
Amm. Cobalto thiocyanate blue
- Method II
- Cobaltous salts form Sod. cobalti carbonate with NaHCO3. Br2 oxidises this complex to Sod. Cobalt carbonate which is apple green and stable towards heat.
CoCl2 + 2NaHCO3  Co(HCO3)2 + 2 NaCl
Co(HCO3)2 + 2 NaCl
Co(HCO3)2 CoCO3 + CO2 + H2O
CoCO3 + CO2 + H2O
CoCO3 + 2NaHCO3  Na4[Co(CO3)3] + 2CO2 + 2H2O
Na4[Co(CO3)3] + 2CO2 + 2H2O
Sod. cobalto carbonate
Br2 + H2O  2HBr + O
2HBr + O
2Na4[Co(CO3)3] + 2NaHCO3 + O  2Na3[Co(CO3)3] + 2Na2CO3 + 2H2O
2Na3[Co(CO3)3] + 2Na2CO3 + 2H2O
(apple green Sod. cobalti carbonate)
(apple green Sod. cobalti carbonate)
- Nickel : Does not form any complex with NaHCO3 but forms black Ni2O3 in alkaline soln of Br2
NiCl2 + 2NaHCO3  Ni(HCO3)2 + 2NaCl
Ni(HCO3)2 + 2NaCl
Ni(HCO3)2  NiCO3 + H2O + CO2
NiCO3 + H2O + CO2
2NaHCO3 + Br2  2NaBr + NaOBr + 2CO2
2NaBr + NaOBr + 2CO2
2NiCO3 + NaOBr + 3H2O  2Ni(OH)3 + NaBr + 2CO2
2Ni(OH)3 + NaBr + 2CO2
2Ni(OH)3 Ni2O3 + 3H2O
Ni2O3 + 3H2O
- Method III
Cobalt salts with a-nitro b - naphthol form brown complex
cobalti nitroso b-naphthol Reddish brown
- Method IV
KCN form the following colourless soluble complexes with Cobalt and Nickel salts.
CoCl2 + 6KCN  K4[Co(CN)6] + 2KCl
K4[Co(CN)6] + 2KCl
K-cobalti cyanide colourless
NiCl2 + 4KCN K2[Ni(CN)4] + 2KCl
K2[Ni(CN)4] + 2KCl
Pot. nickelo cyanide colourless
If the soln is treated with sod. hypobromite (Br2 in presence of NaOH) the Ni cyanide is decomposed and black ppt of Ni2O3 is obtained
Br2 + 2NaOH NaBr + NaOBr + H2O
NaOBr  NaBr + O
NaBr + O
2K2[Ni(CN)4] + 4NaOH + O Ni2O3 + 4NaCN +4KCN + 2H2O
Ni2O3 + 4NaCN +4KCN + 2H2O
Black
And K. cobalto cyanide is oxidised to K.cobalti cyanide which is still colourless
4K4[Co(CN)6] + 2H2O + 2O 4K3[Co(CN)6] +4KOH
K-cobalti cyanide colourless
- Method V
When KNO2 is added to a cobaltous salt soln in presence of acetic acid a bright yellow ppt of K-cobaltinitrite is obtained.
CoCl2 + 2KNO2 Co(NO2)2 + KCl
Co(NO2)2 + KCl
KNO2 + CH3COOH CH3COOK + HNO2
CH3COOK + HNO2
Co(NO2)2 + 3 KNO2 + 2HNO2  K3[Co(NO2)6] + NO + H2O
K3[Co(NO2)6] + NO + H2O
K- cobaltinitrite yellow ppt
The corresponding sod. salt is soluble in H2O and hence reagent for K+ ions. Nickel does not form any ppt in presence of CH3COOH acid
SOME PRECAUTIONS FOR GROUP IV ANALYSIS
- H2S must be passed in hot soln otherwise ZnS and MnS will form colloidal soln.
- H2S should not be passed for a long time otherwise NiS will turn to colloidal solution
- The NH4Cl checks the formation of colloidal soln.
- KCNS cannot be used in place of NH4CNS in test of Co(see method I above)
- After dissolving sulphides in dil HCl the H2S should be completely removed by boiling the solution before adding NaOH soln otherwise ZnS will be ppted and may be confused for Mn(OH)2
- When both Co and Ni are present then the soln is first treated with little NH4OH and green Co(OH)2 is removed by filtration. The test for Ni with DMG should be performed in the filtrate because cobalt interferes in the Ni test reacting with DMG to form brown coloured complex ion.
DETECTION OF BASIC RADICALS - GROUP V
Member : Ba++, Sr++ Ca++ ppted as carbonates
Group reagent : (NH4)2CO3 in presence of NH4Cl and NH4OH
- High concentration of NH4Cl prevents complete precipitation of the carbonates of this group.
- Before adding (NH4)2CO3 the volume of the solution must be reduced to one-third by evaporation because the solution becomes very dilute upto Vth group.
- Barium chromate can not be precipitated in presence of an appreciable hydrogen ion concentration, therefore acetic acid is used and that too in minimum possible amount.
- It is better to use saturated solution of (NH4)2SO4 to detect Sr++ radical. After addition of the reagent, wait for few minutes to see if a precipitate of SrSO4 is obtained.
- The solubility product of SrSO4 is sufficiently large which may result in its incomplete precipitation. Sr++ ions may, therefore, co-precipitate as SrC2O4 along with CaC2O4
- From concentrated solutions calcium may get co-precipitated as calcium sulphate with strontium sulphate.
- Sometimes calcium does not get precipitated in its group due to the formation of Ca(HCO3)2 on addition of (NH4)2CO3 to the concentrated filtrate of Vth group as Ca(HCO3)2 is soluble and so it passes into the filtrate of Vth group. Hence filtrate of Vth group must be tested for calcium before proceeding to VIth group of the analysis.
- BaCrO4 is not sufficiently volatile to give flame test therefore it should be converted to chloride.
- The insoluble carbonate of Ba, Sr and Ca are precipitated on the addition of (NH4)2CO3 to the filtrate of group IV.
- Barium chromate is insoluble in acetic acid while the chromates of Sr and Ca are soluble in it.
- Ammonium sulphate brings about the precipitation of SrSO4 only while calcium is not precipitated by this reagent due to the formation of complex salt
(NH4)2 [Ca(SO4)2]. - Calcium oxalate being insoluble in acetic acid, is precipitated by the addition of ammonium oxalate.
BARIUM (Ba++)
- BaCl2 + (NH4)2CO3
BaCO3 ¯ + 2NH4Cl white ppt
BaCO3 + 2CH3COOH (CH3COO)2Ba + H2O+CO2
(CH3COO)2Ba + H2O+CO2
- Pot. chromate :
(CH3COO)2Ba + K2CrO4 BaCrO4 ¯ + 2CH3COOK yellow
BaCrO4 ¯ + 2CH3COOK yellow
Soluble in mineral acids but insoluble in CH3COOH.
Chromates of Sr and Ca are soluble in acetic acid
(CH3COO)2Ba + K2CrO4  BaCrO4 + 2CH3COOK
BaCrO4 + 2CH3COOK
- H2SO4 : BaCl2 + H2SO4
BaSO4 ¯ + 2HCl
- Amm. oxalate : BaCl2 + (NH4)2C2O4
BaC2O4 + 2 NH4Cl
White ppt readily soluble in CH3COOH
STRONTIUM (Sr++)
- (NH4)2CO3 : SrCl2 + (NH4)2CO3
SrCO3 + NH4Cl white ppt
SrCO3 + 2CH3COOH  (CH3COO)2Sr + H2O + CO2 soluble
(CH3COO)2Sr + H2O + CO2 soluble
On adding K2CrO4 for the detection of Ba SrCrO4 is not ppted in presence of acetic acid.
Sr(CH3COO)2 + K2CrO4 SrCrO4 + 2CH3COOK soluble
SrCrO4 + 2CH3COOK soluble
- (NH4)2SO4 : SrCrO4+ (NH4)2SO4
SrSO4 + (NH4)2CrO4
white ppt, Ca remain in the solution as soluble complex. - Amm oxalate Soln : SrCrO4+ (NH4)2C2O4
SrC2O4 + (NH4)2CrO4
white ppt insoluble in acetic acid
CALCIUM (Ca++)
- (NH4)2CO3 : CaCl2+ (NH4)2CO3
CaCO3 + 2NH4Cl white ppt
CaCO3+ 2CH3COOH  (CH3COO)2Ca + CO2 + H2O soluble.
(CH3COO)2Ca + CO2 + H2O soluble.
On adding K2CrO4, Calcium chromate is not ppted but remains in the soln
Ca(CH3COO)2 + K2CrO4  CaCrO4 + 2CH3COOK Soluble
CaCrO4 + 2CH3COOK Soluble
On adding (NH4)2SO4 to CaCrO4; CaSO4 is not ppted but soluble complex is formed..
CaCrO4 + 2(NH4)2SO4 (NH4)2[Ca(SO4)2] + NH4CrO4 Soluble complex
(NH4)2[Ca(SO4)2] + NH4CrO4 Soluble complex
- Amm oxalate : (NH4)2[Ca(SO4)2] + (NH4)2C2O4
CaC2O4 + (NH4)2SO4
White ppt insoluble in acetic acid but soluble in mineral acids.
- K4[Fe(CN)6]Soln : CaCl2 + K4 [Fe(CN)6]
Ca.K2[Fe(CN)6]
+ 2KCl
white ppt
DETECTION OF BASIC RADICALS - GROUP VI
MAGNESIUM (Mg++)
- Sod. hydrogen phosphate soln :
Mg(NO3)2 + Na2HPO4+ NH4OH  Mg(NH4)PO4+ 2NaNO3 + H2O
Mg(NH4)PO4+ 2NaNO3 + H2O
White ppt.
- NH4OH :
MgCl2 + 2NH4OH  Mg(OH)2 + 2NH4Cl
Mg(OH)2 + 2NH4Cl
white ppt. soluble in excess of NH4Cl
AMMONIUM (NH4+)
- NaOH :
NH4Cl + NaOHNH3 + H2O + NaCl
NH3 + HCl  NH4Cl White fumes
NH4Cl White fumes
- Nessler’s reagent :
2KI +HgCl2 HgI2 + 2KCl
HgI2 + 2KI  K2[HgI4]
K2[HgI4]
2K2[HgI4] + NH4OH + 3NaOH 
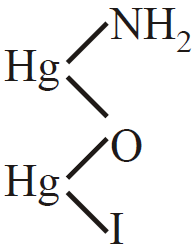 + 4KI + 3NaI + 3H2O
+ 4KI + 3NaI + 3H2O
Iodide of millon’s base (Brown ppt)
- Mercurous nitrate : Hg2(NO3)2 + 2NH3
+ NH4NO2
- CuSO4 : CuSO4 + 4NH3
[Cu(NH3)4]SO4
deep blue
SODIUM (Na+)
- Pot. dihydrogen antimonate :
KH2SbO4 +NaCl  NaH2SbO4 +KCl
NaH2SbO4 +KCl
white ppt
- Uranyl Mg. acetate solution :
NaCl + 3UO2(CH3COO)2 + Mg(CH3COO)2 + CH3COOH
 HCl + NaMg(UO2)3 (CH3COO)9 yellow ppt
HCl + NaMg(UO2)3 (CH3COO)9 yellow ppt
- Uranyl zinc acetate reagent :
Yellow crystalline Sod. Zinc acetate NaZn(UO2)3  (CH3COO)9 9H2O
(CH3COO)9 9H2O
POTASSIUM (K+)
- Chloroplatinic acid : Golden yellow crystalline ppt. in neutral or acid medium.
- Sod. Cobaltinitrite :
Na3[Co(NO2)6] + 3KCl  K3[Co(NO2)6] + 3NaCl
K3[Co(NO2)6] + 3NaCl
K.cobaltinitrite yellow ppt. (fischer’s salt)
- Sod. hydrogen tartrate : In conc. solution the reagent in the presence of alcohol gives a white ppt on scratching the sides with a glass rod
2KCl+H2PtCl6  K2PtCl6+ 2HCl
K2PtCl6+ 2HCl
ACTION OF HEAT ON SOME INORGANIC COMPOUNDS
CARBONATES
Except all alkali metal carbonates are stable.
all alkali metal carbonates are stable.
smelling salt
2Ag2CO3 4Ag + 2CO2+ O2
4Ag + 2CO2+ O2
2Hg2CO3  4 Hg + 2
4 Hg + 2 +
+ 
Rest – give metal oxide and 
NITRATES
Except all alkali metal nitrates decompose to nitrites and .
Na, K, Rb, Cs 
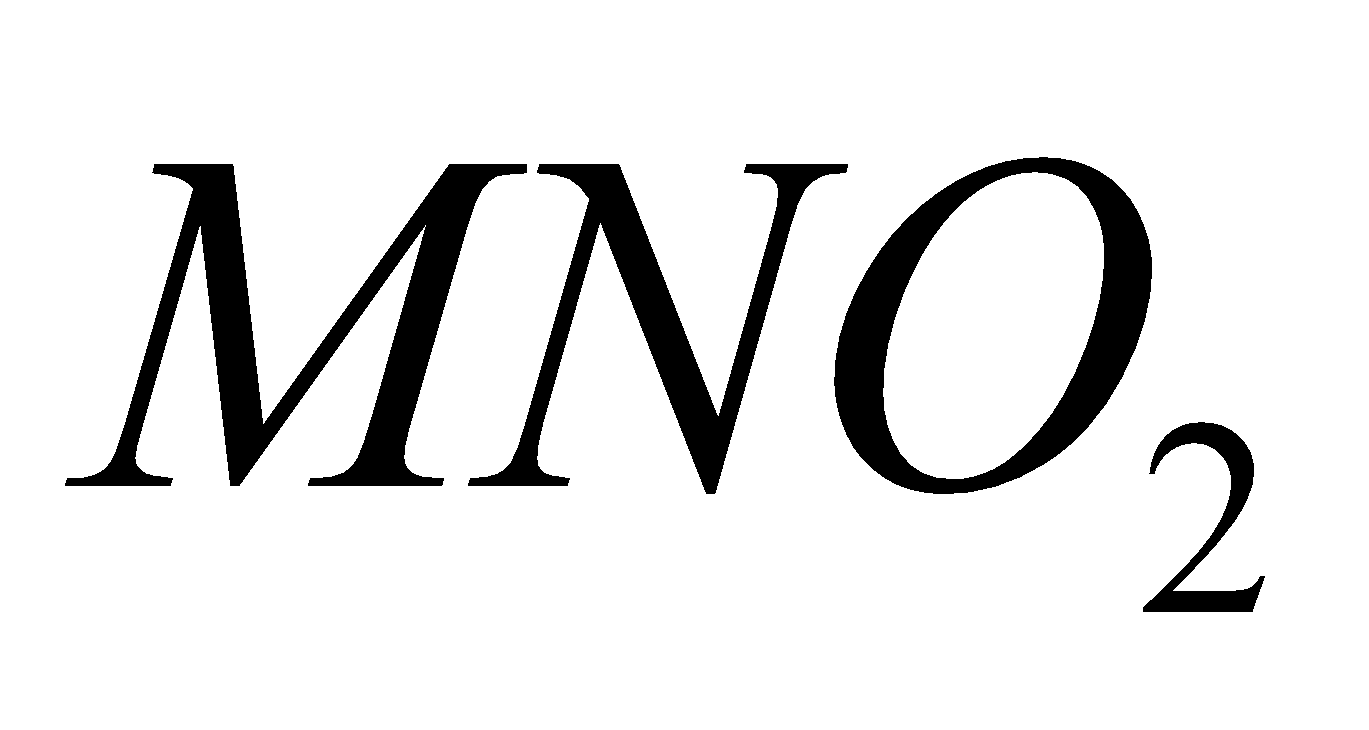 +
+ 
4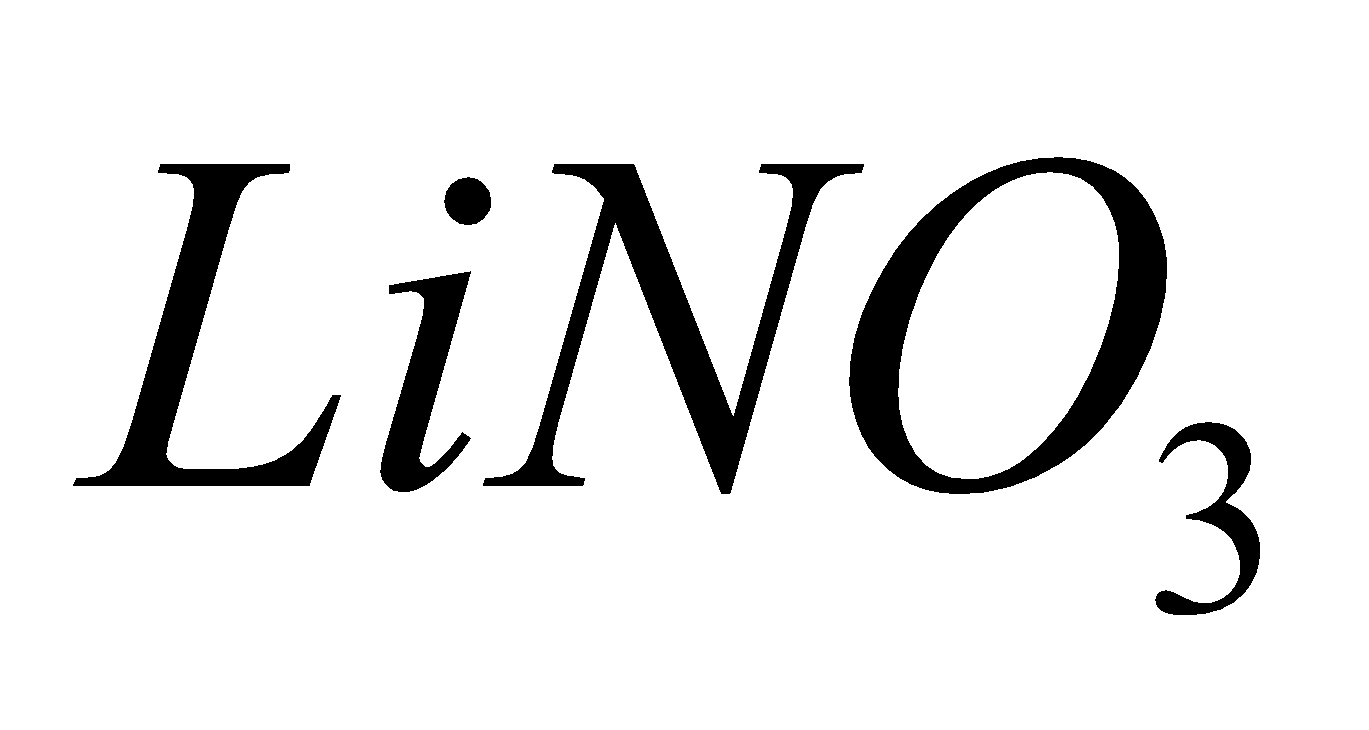
 2
2 + 4
+ 4 +
+ 
2Ag
 2 Ag + 2
2 Ag + 2 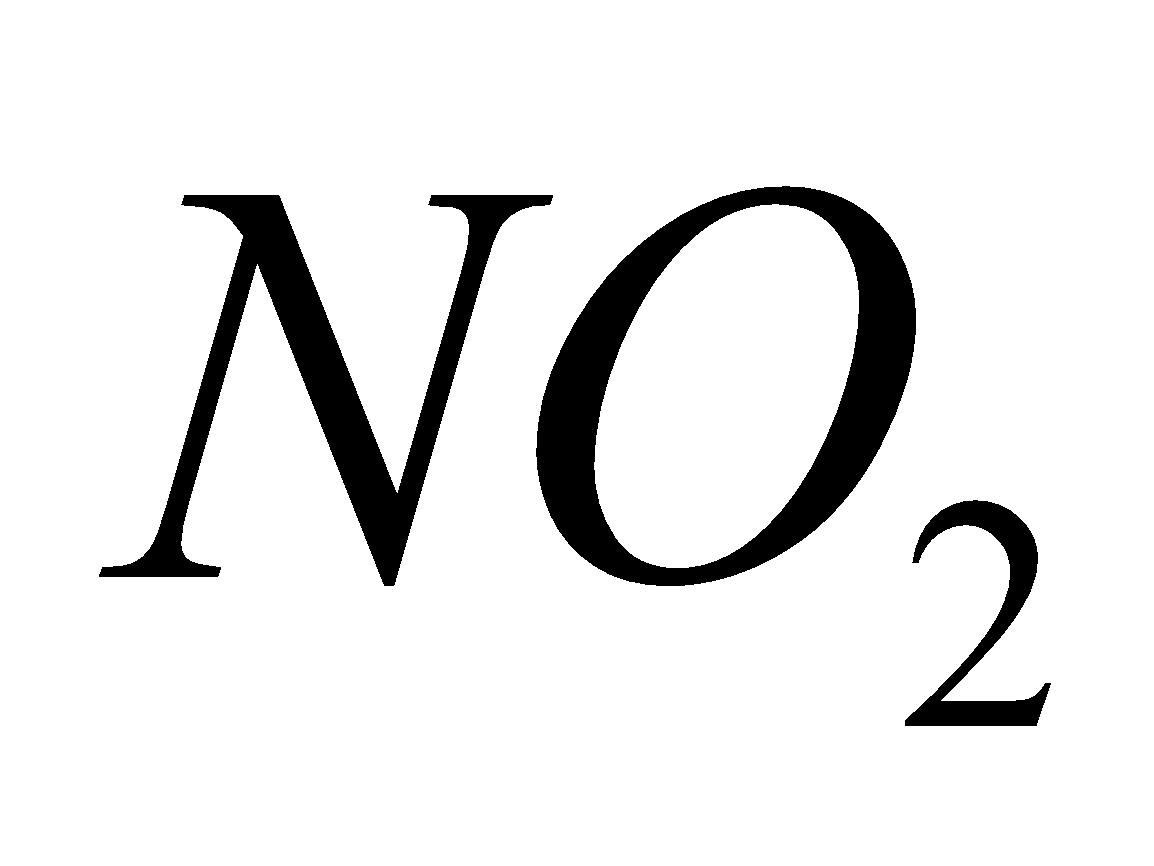 +
+ 
Hg2
 2 Hg + 2
2 Hg + 2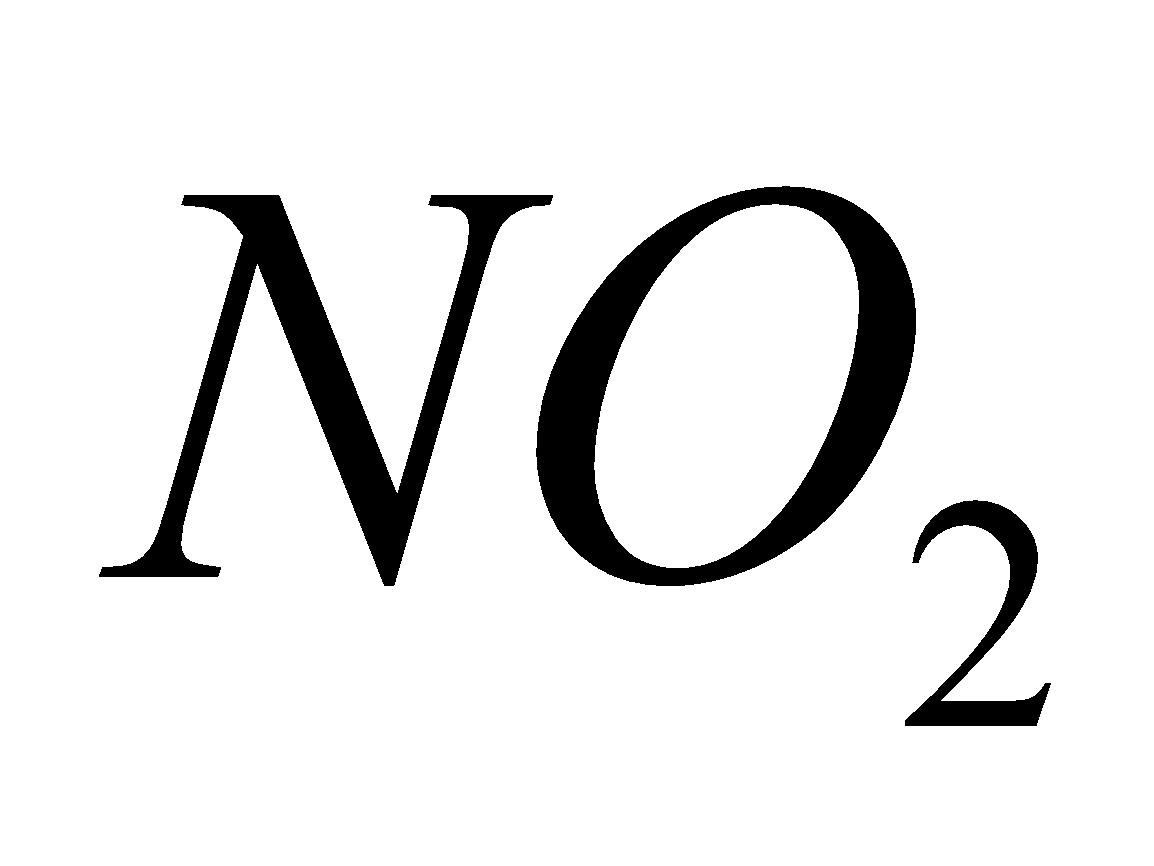 +
+ 
NH4NO3  2
2 +
+ 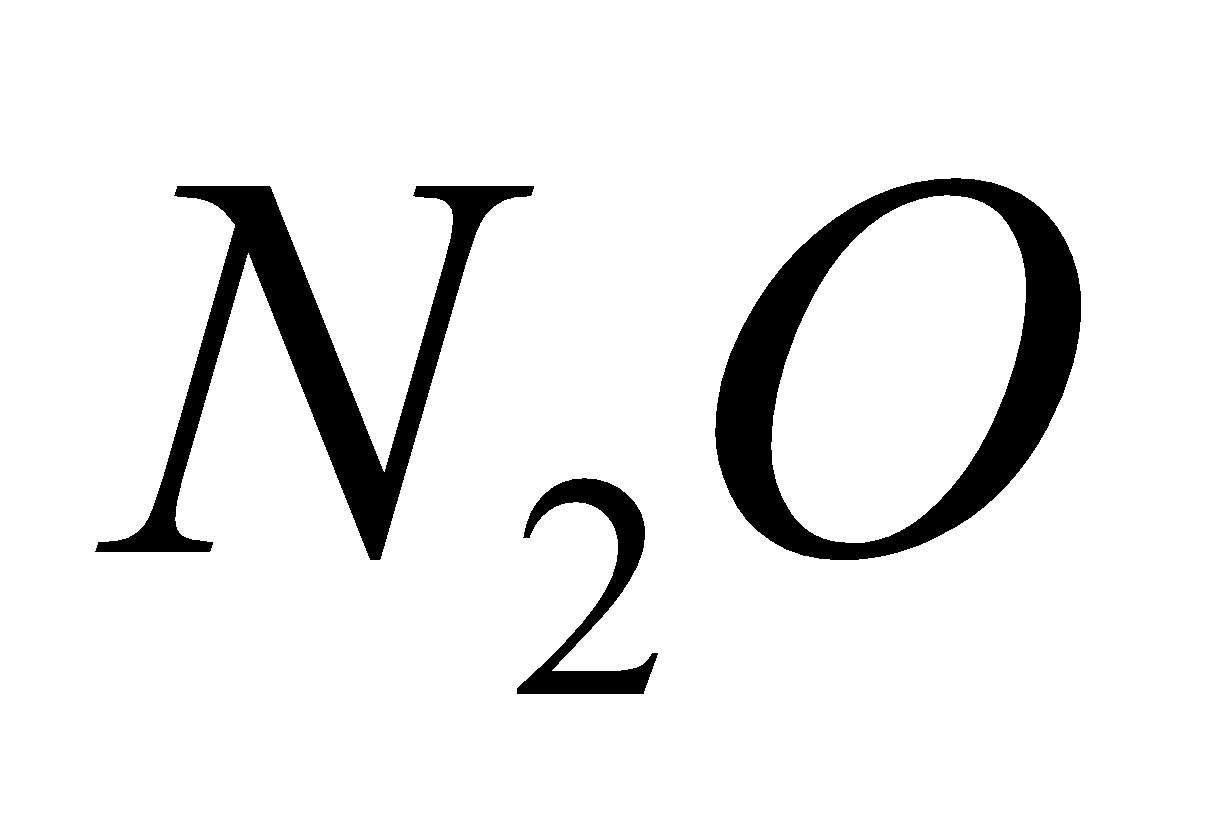 (laughing gas)
(laughing gas)
Rest  metal oxide +
metal oxide +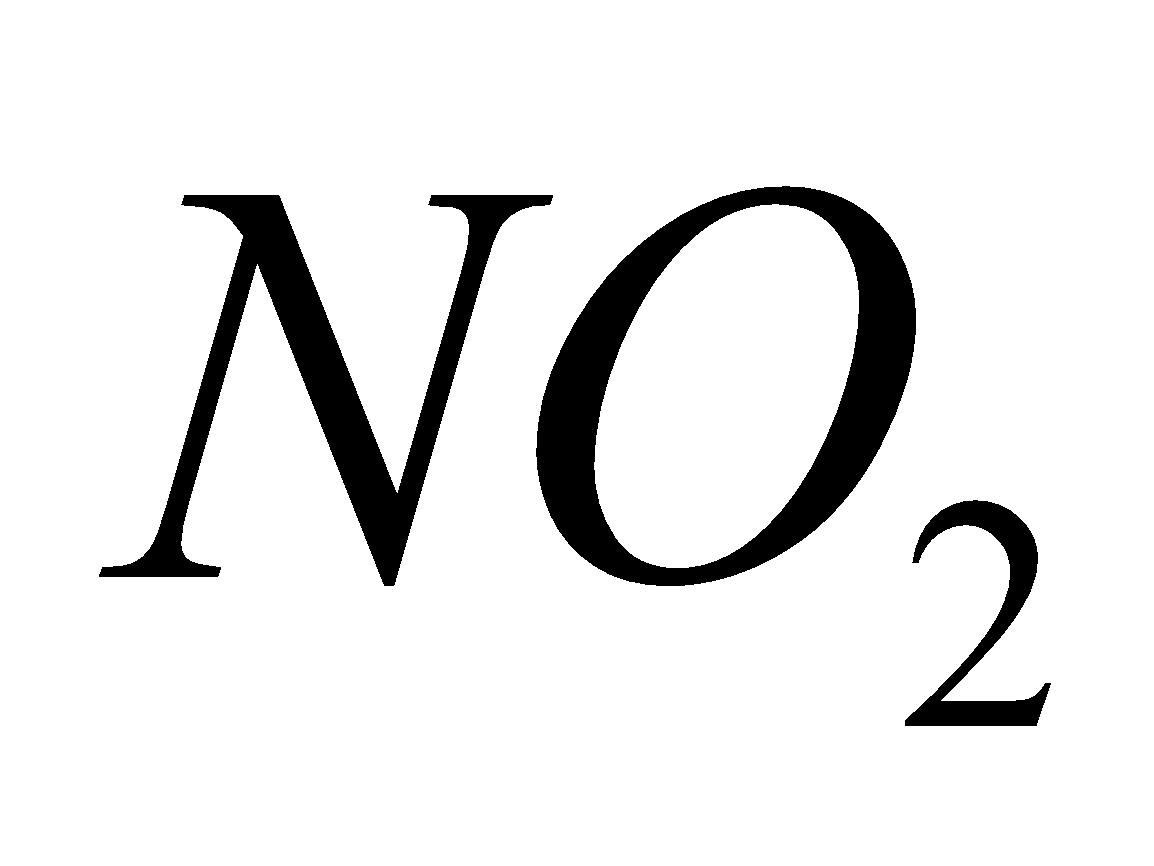 +
+ 
SULPHATES
Alkali metal sulphates are stable to heat. On heating they lose water of crystallisation.
Glauber’s salt salt cake
Anhydride
Green vitriol white Red brown
3Hg
 + Hg + 2 SO2 + 2
+ Hg + 2 SO2 + 2
BICARBONATES
On heating give 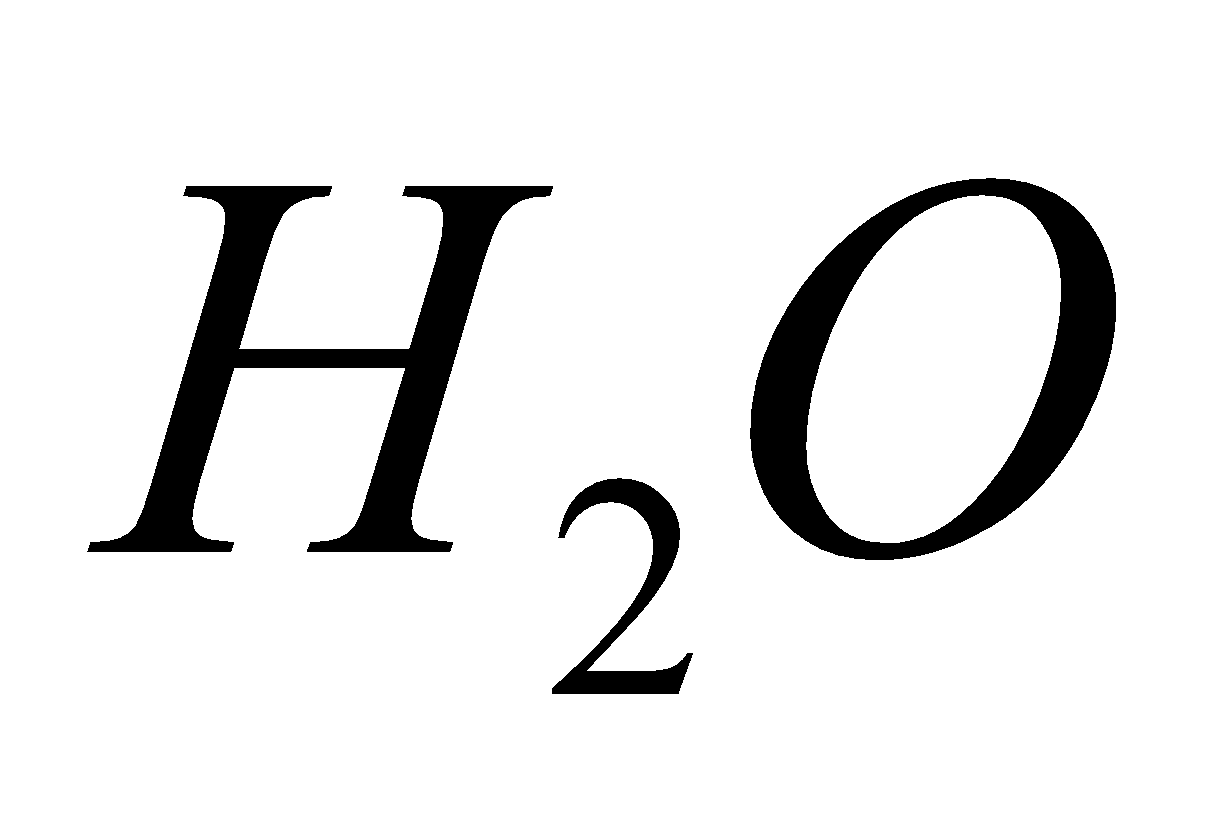 +
+ 
2

 +
+  +
+ 
Ca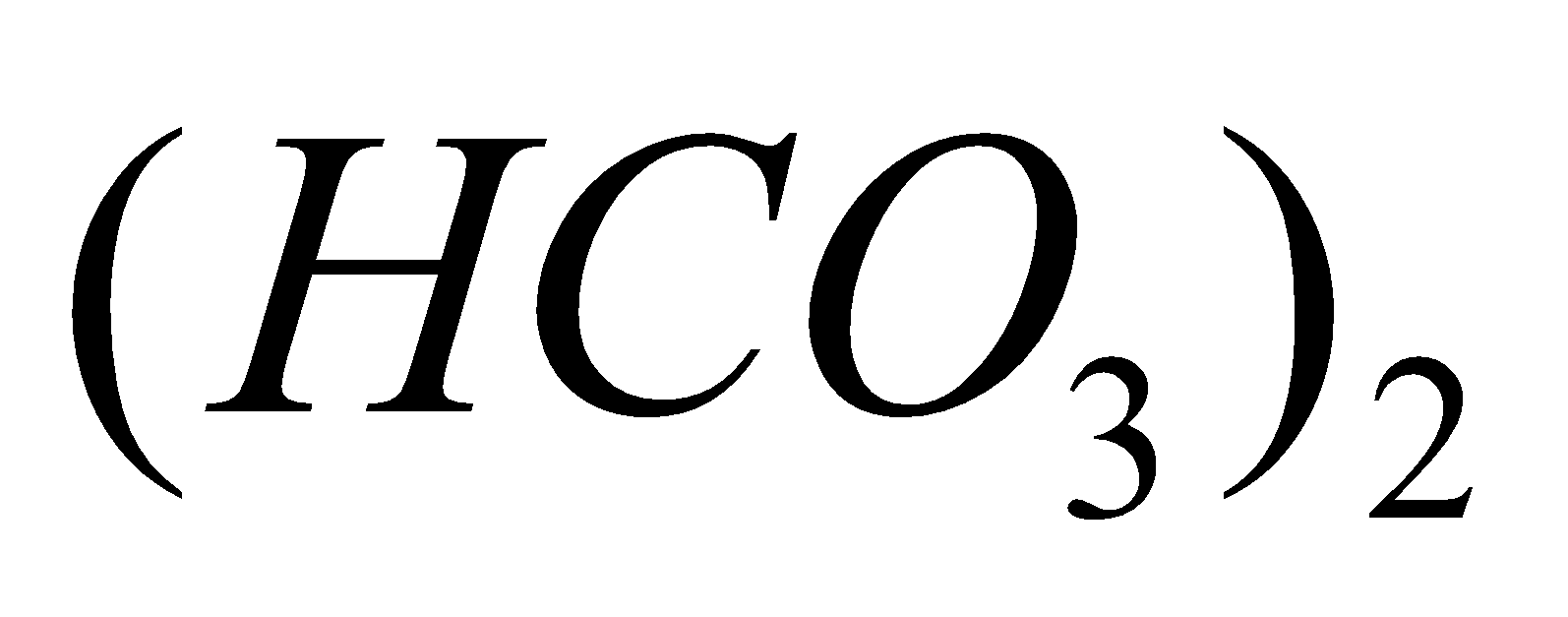

 +
+ 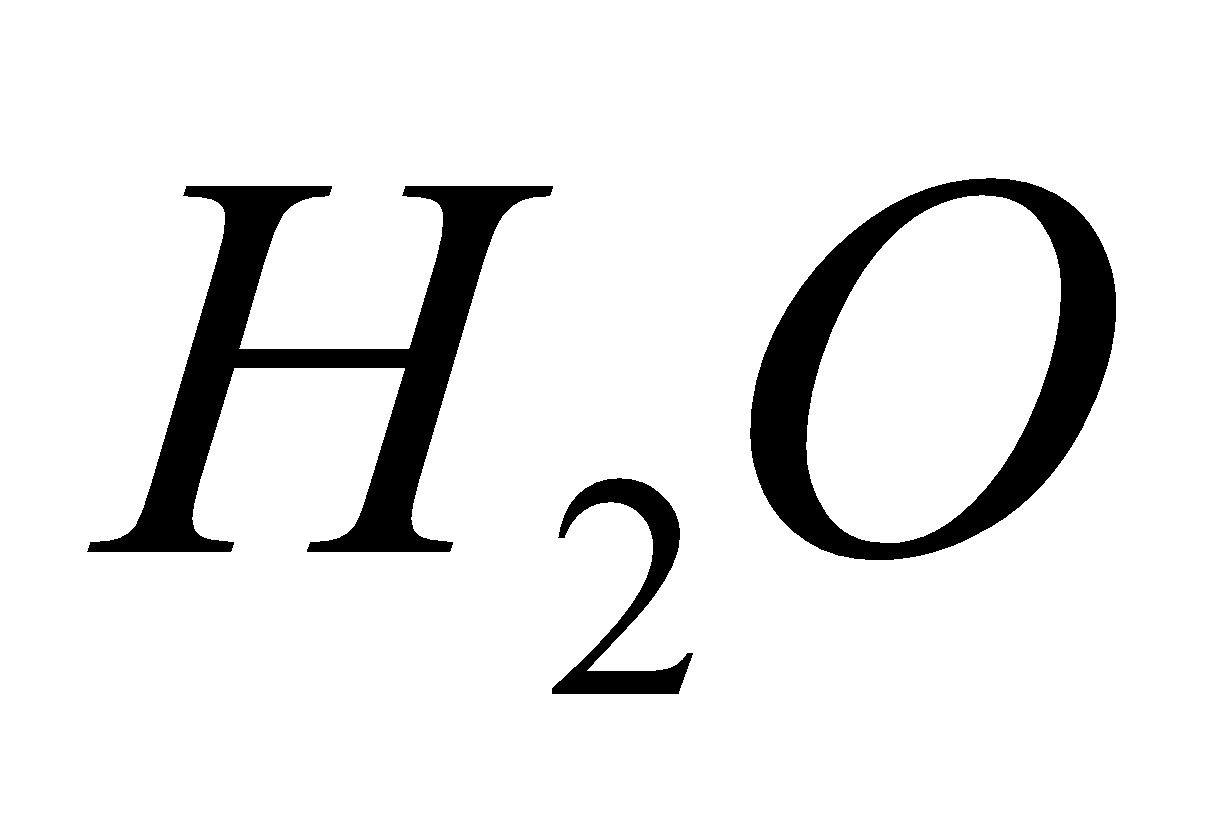 +
+ 
CHLORIDES
Ammonium chloride
2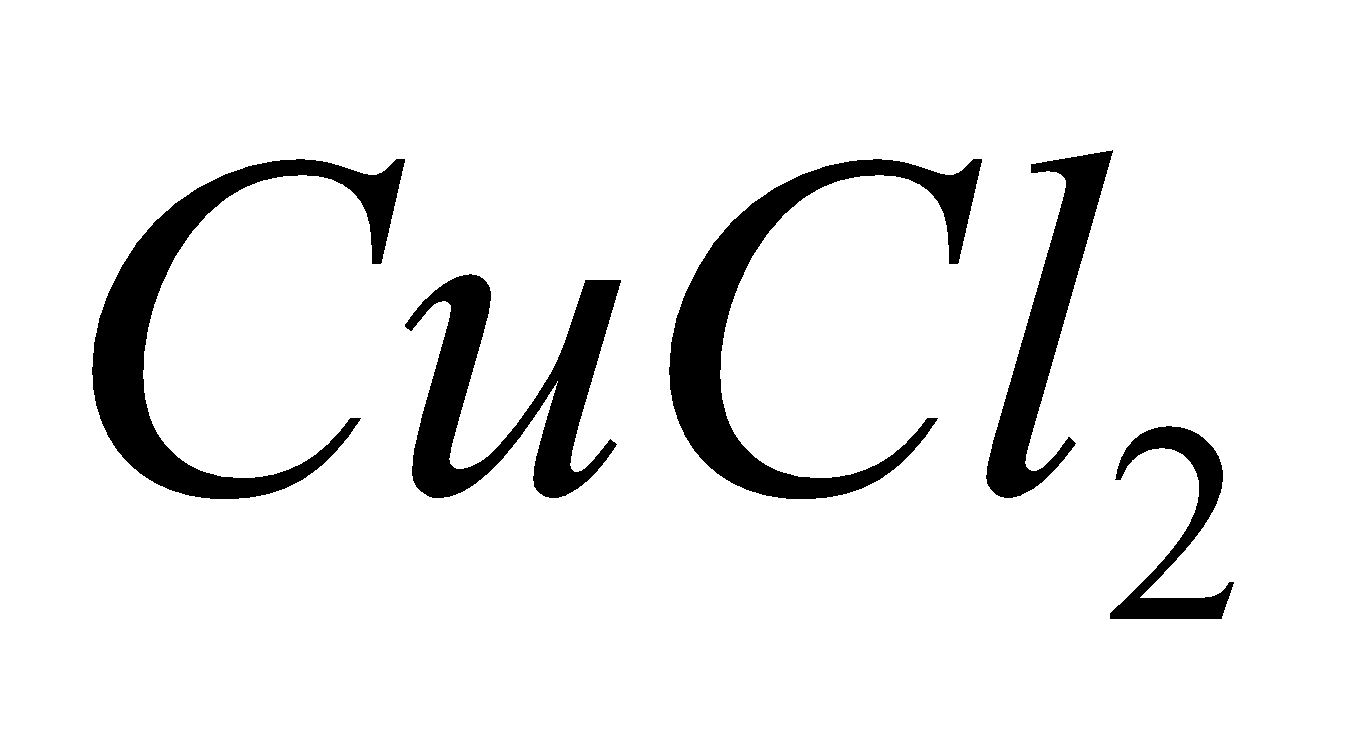

 +
+ 
Zn.2
 Zn(OH)Cl + HCl +
Zn(OH)Cl + HCl + 
2Zn(OH)Cl  Zn2O +
Zn2O + 
Mg.6
 Mg(OH)Cl + HCl + 5
Mg(OH)Cl + HCl + 5
Mg(OH)Cl  MgO + HCl
MgO + HCl
Mg.6
 Mg
Mg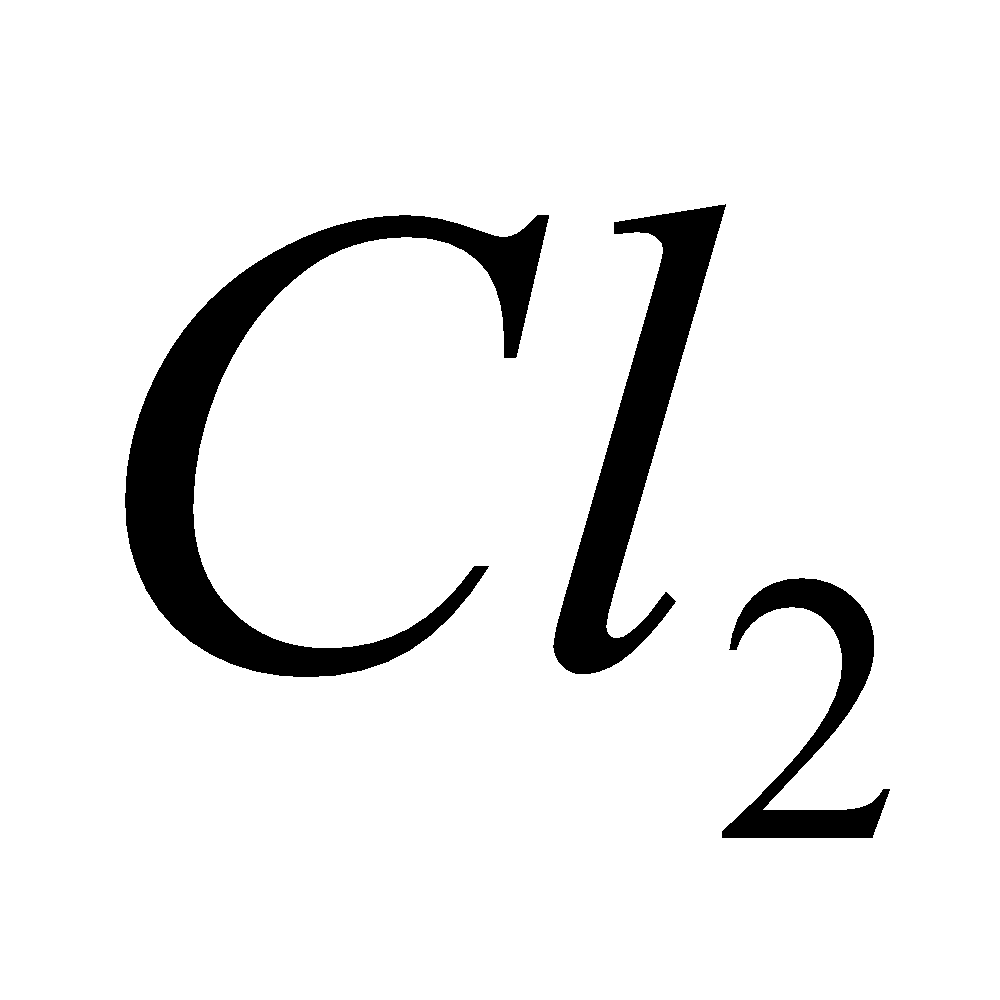 + 6
+ 6
2 
 2
2 +
+ 
Calomel Corrosive sublimate
IODIDES
2Cu 

 +
+ 
PHOSPHATES
Sod. pr. phosphate sod. meta phosphate
2Mg


 + 2
+ 2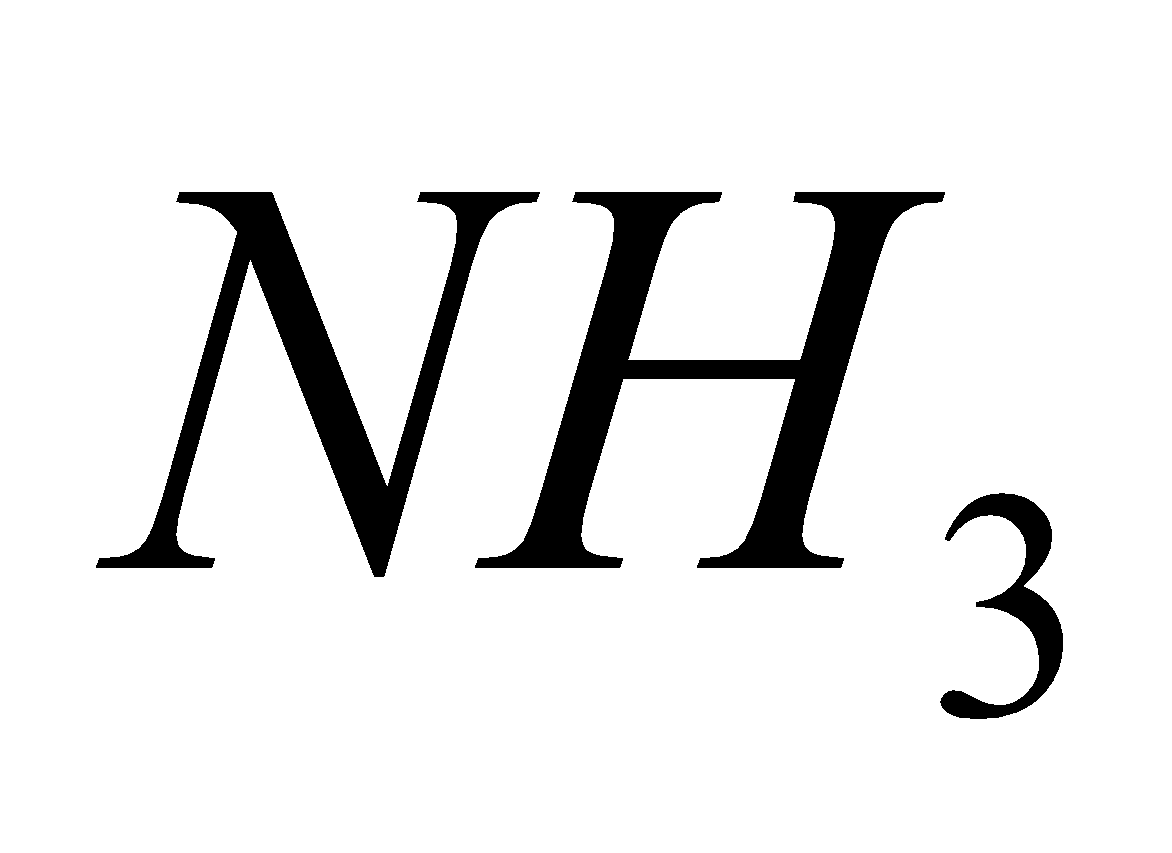 +
+ 
Mg. Pyrophosphate
Na(NH4)HPO4.4H2O  Na(NH4)HPO4 + 4H2O
Na(NH4)HPO4 + 4H2O Na
Na  +
+ +
+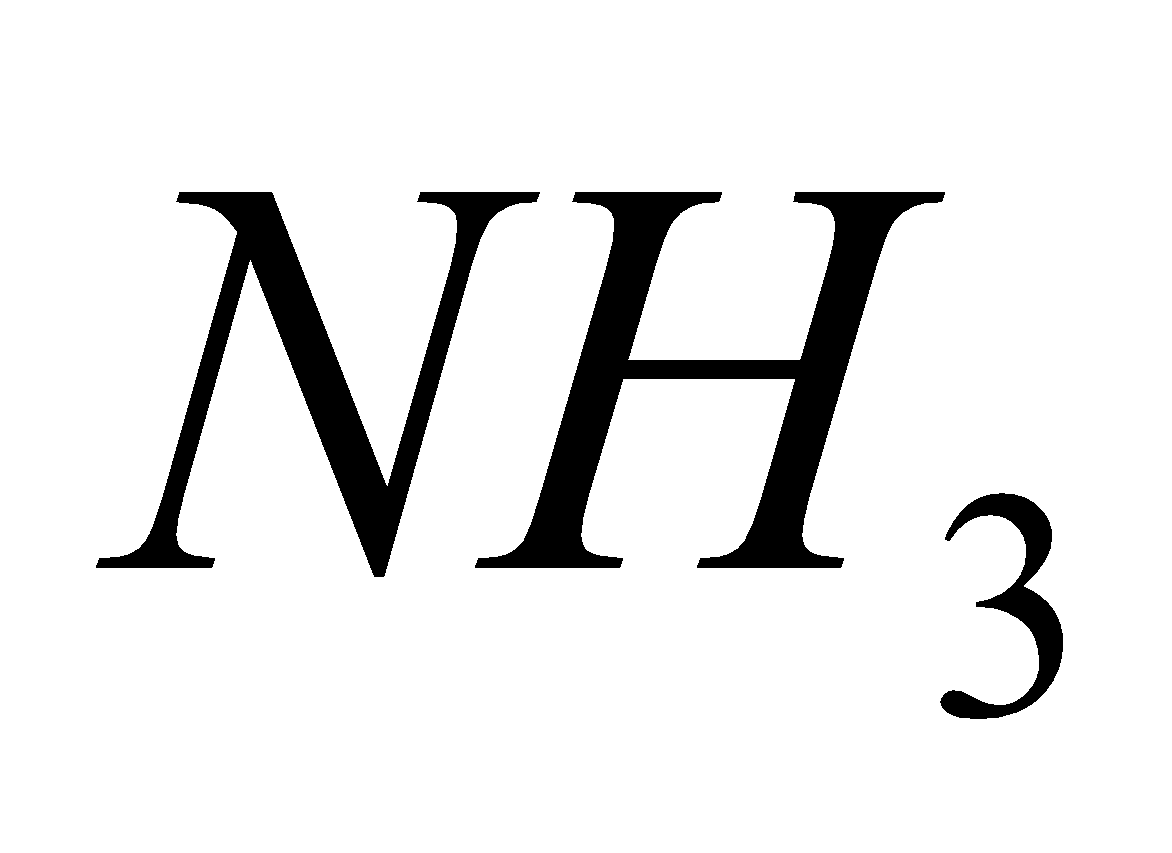
Microcosmic salt Sod. meta phosphate (Glassy Bead)
[CuO + Na
 CuNa PO4 ]
CuNa PO4 ]
Blue bead
2

 +
+ 
 2H
2H +
+
Orthophosphorus acid Pyrophosphoric acid Metaphosphoric acid
2 

 +
+ 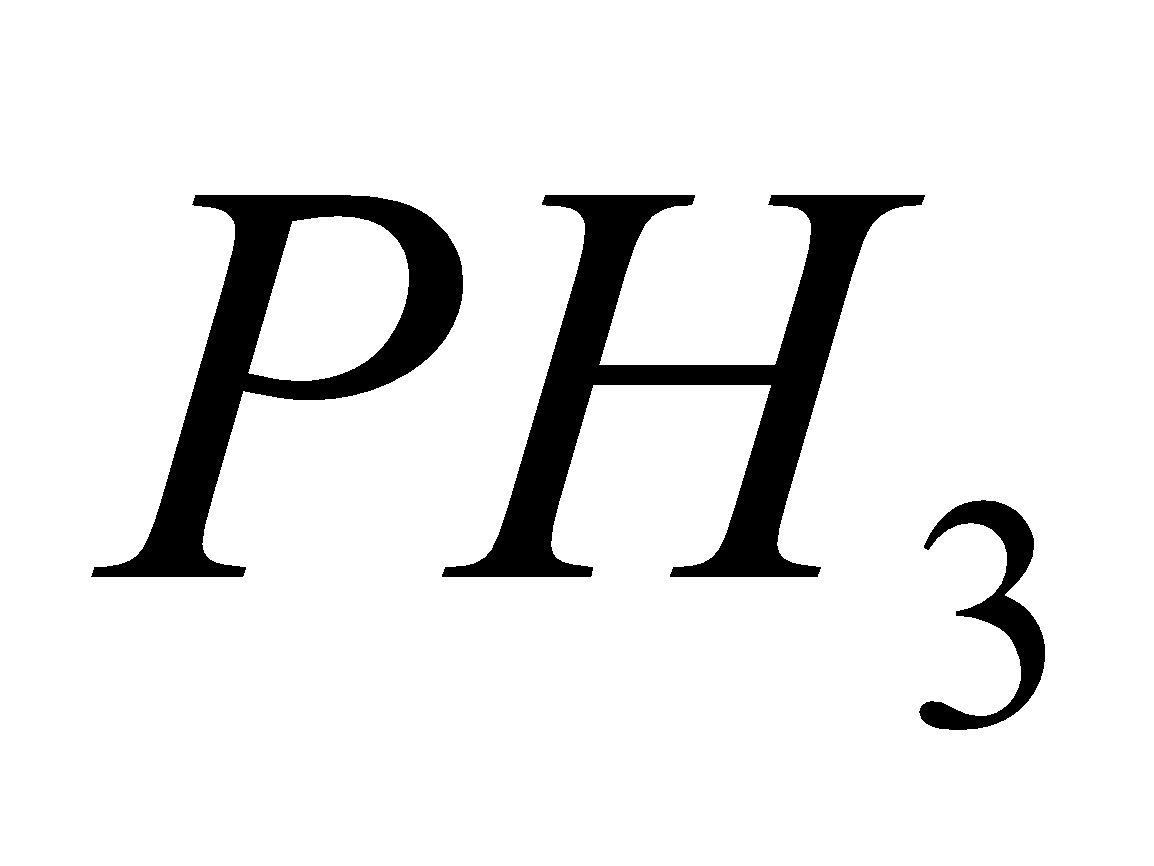
Hypophosphorous acid
4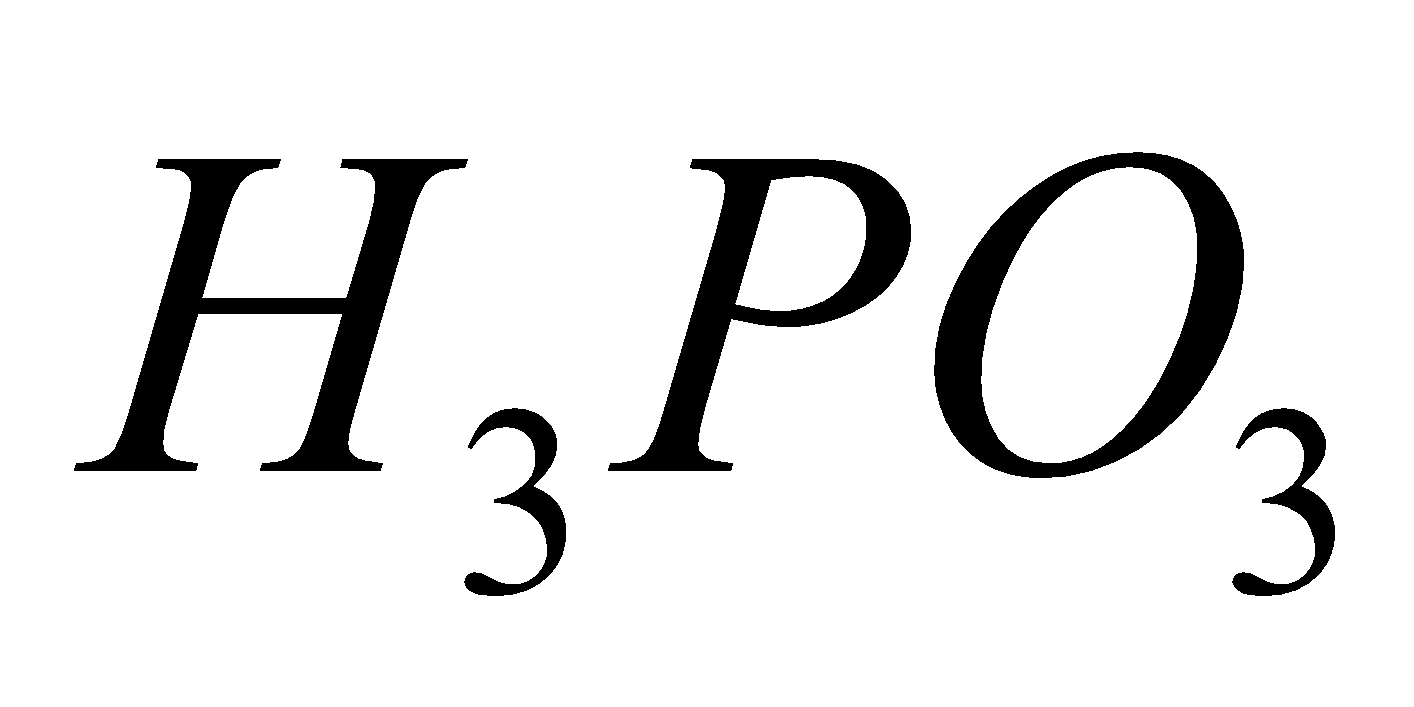
 3
3 +
+ 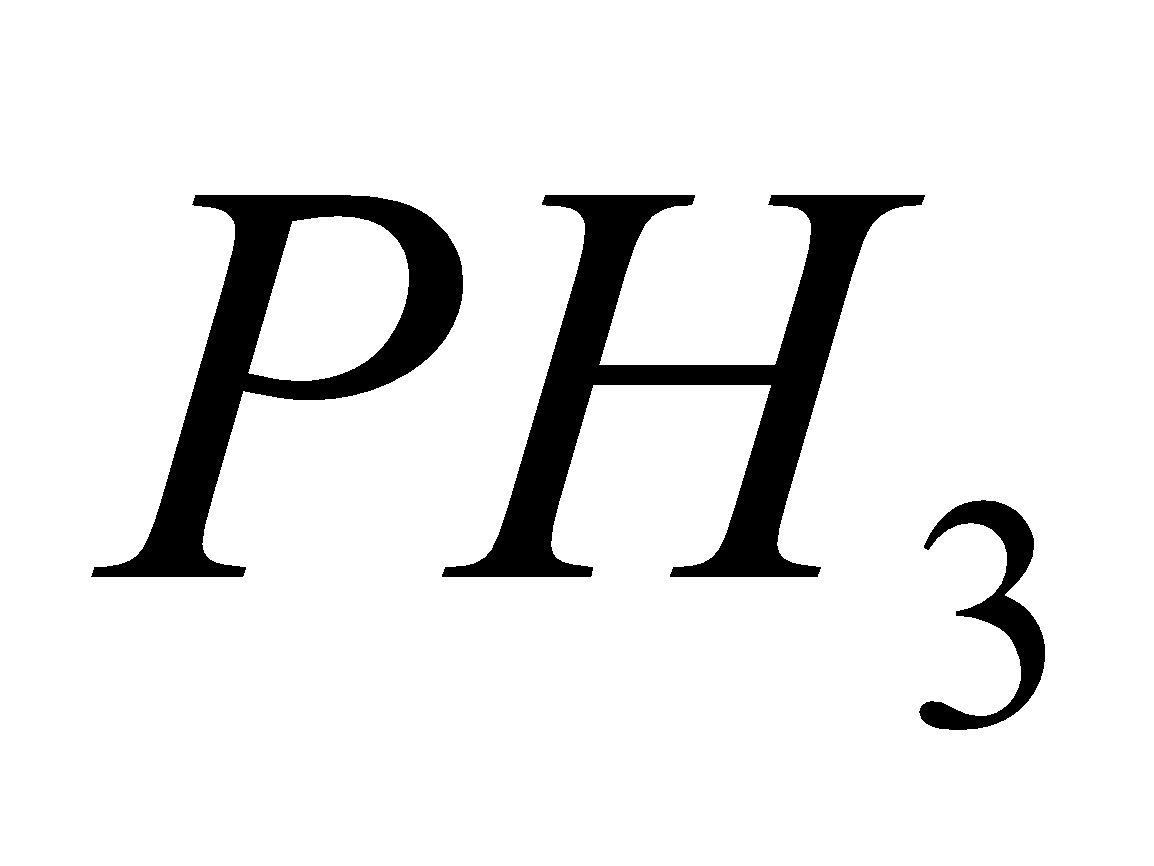
Orthophosphorus acid
OXIDES
Minimum Red lead, litharge
2HgO  2 Hg +
2 Hg + 
ZnO (White)  ZnO (Yellow)
ZnO (Yellow)
CHROMATE / DICHROMATE


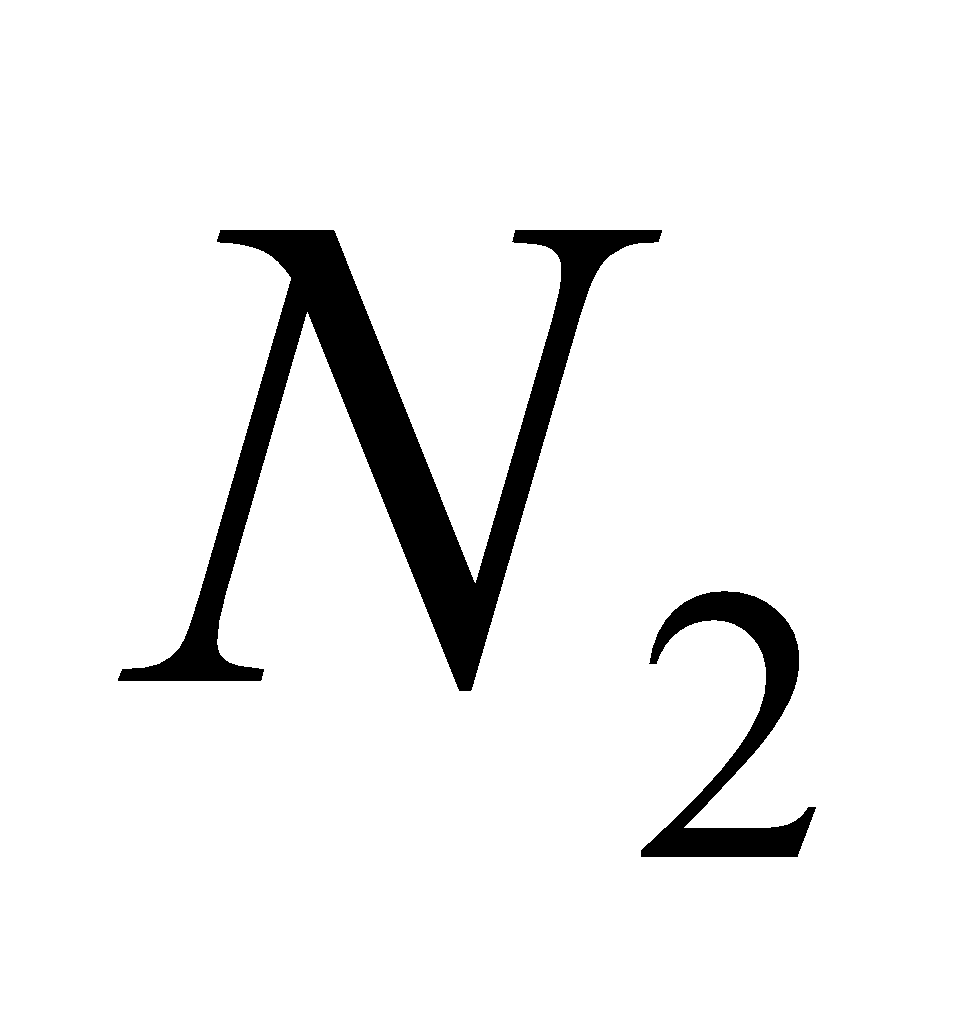 +
+  + 4
+ 4
4
 4
4 + 2
+ 2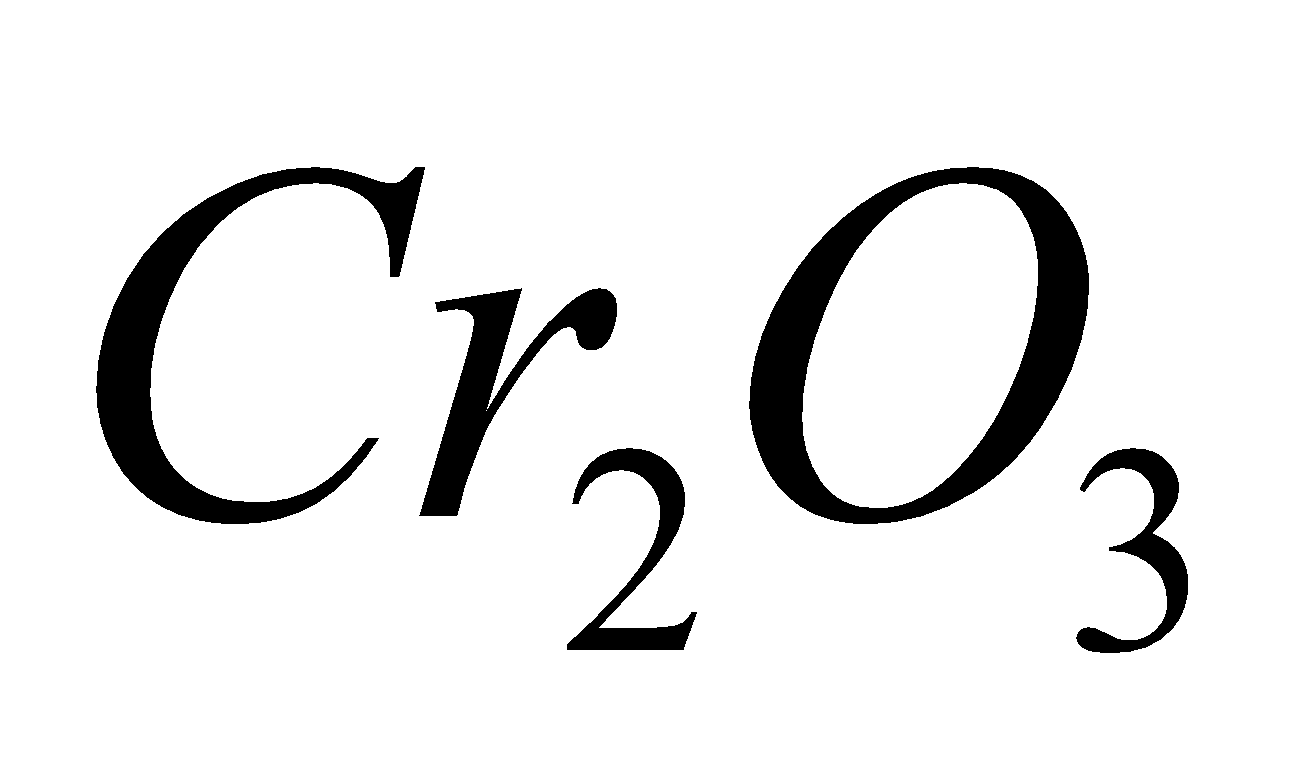 + 3
+ 3
Green
PERCHLORATE
4KCl +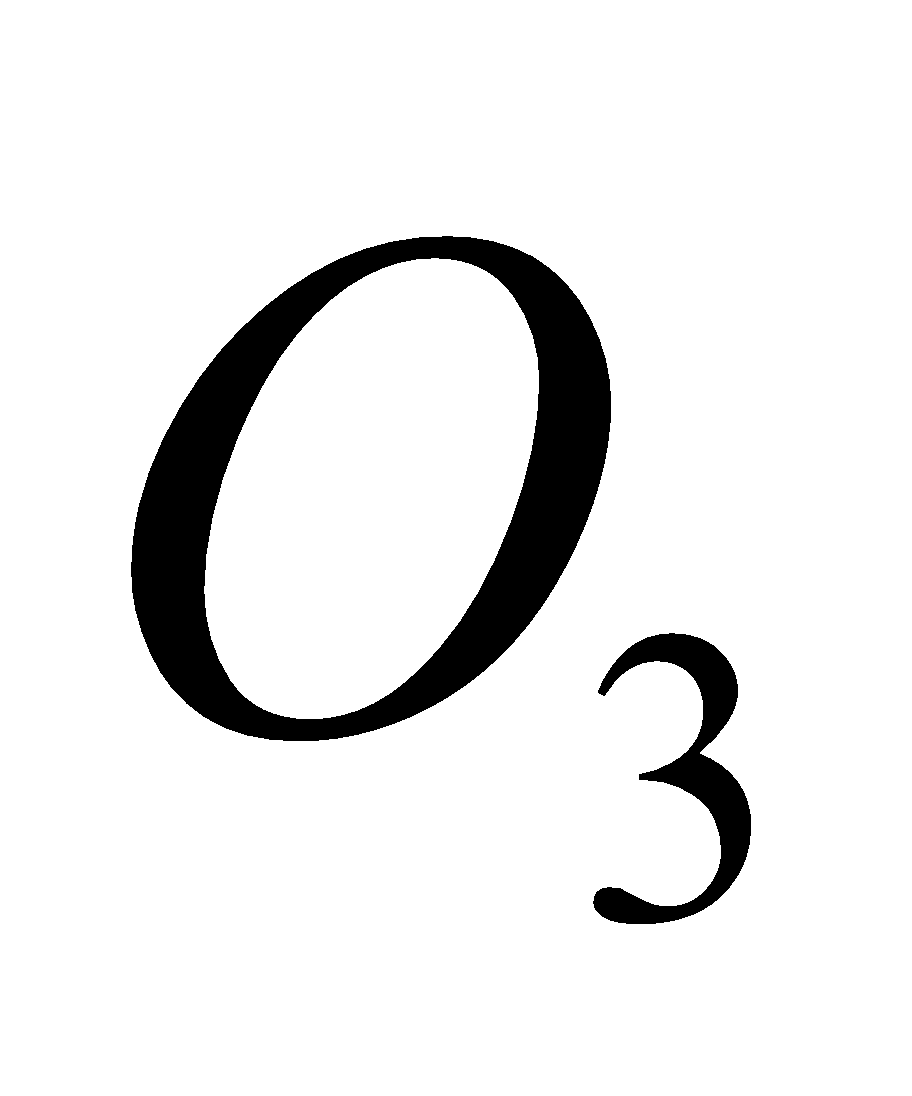
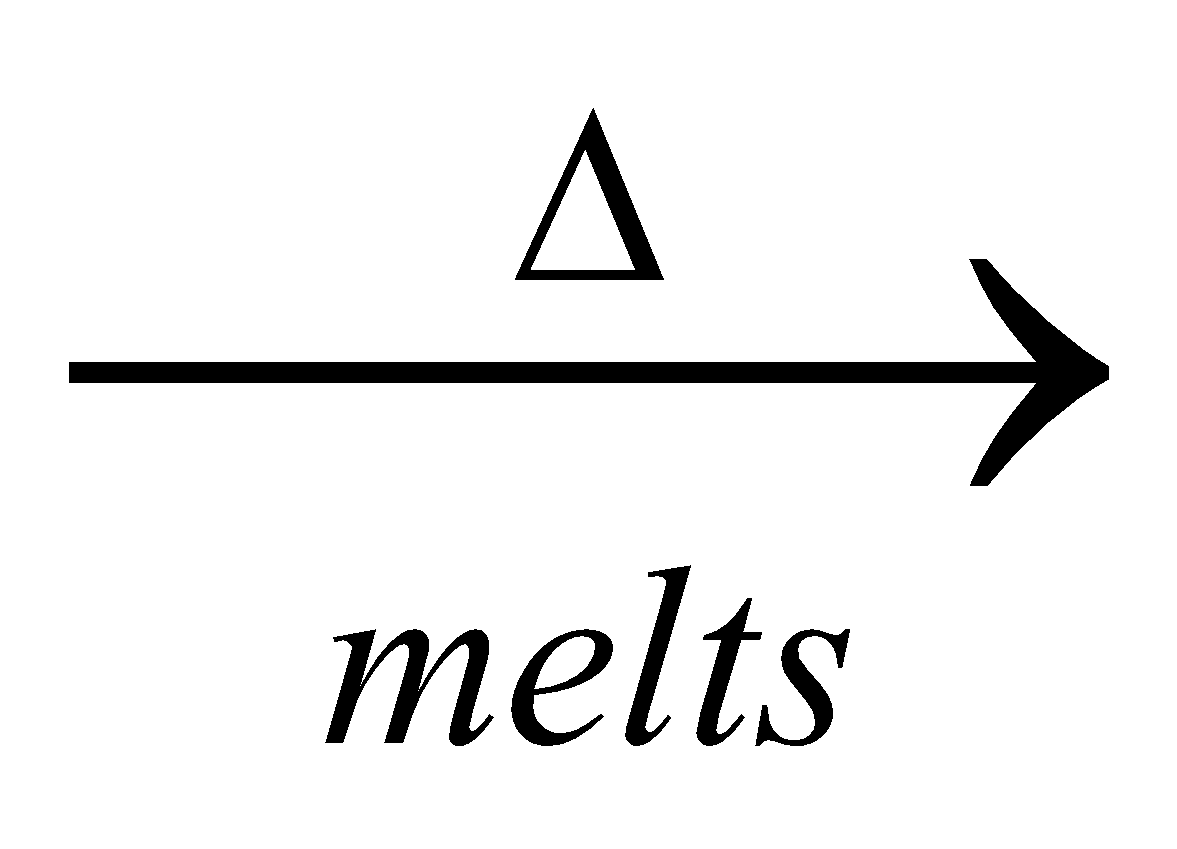 2
2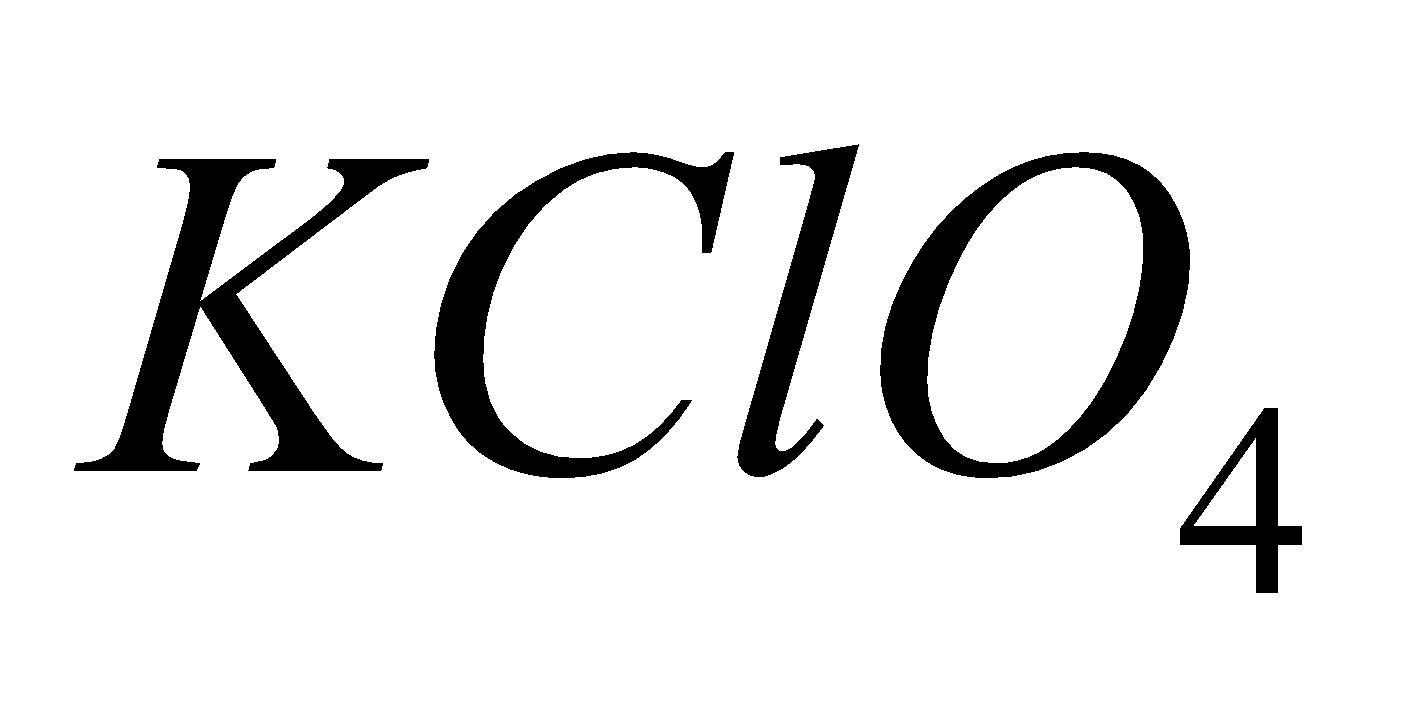 + 2KCl
+ 2KCl
BORATE
Borax Swelled Borax Glassy Residue
Orthoboric acid Metaboric acid Tetraboric acid Boric anhydride (glassy residue)
CYANATE



MANGANATE
2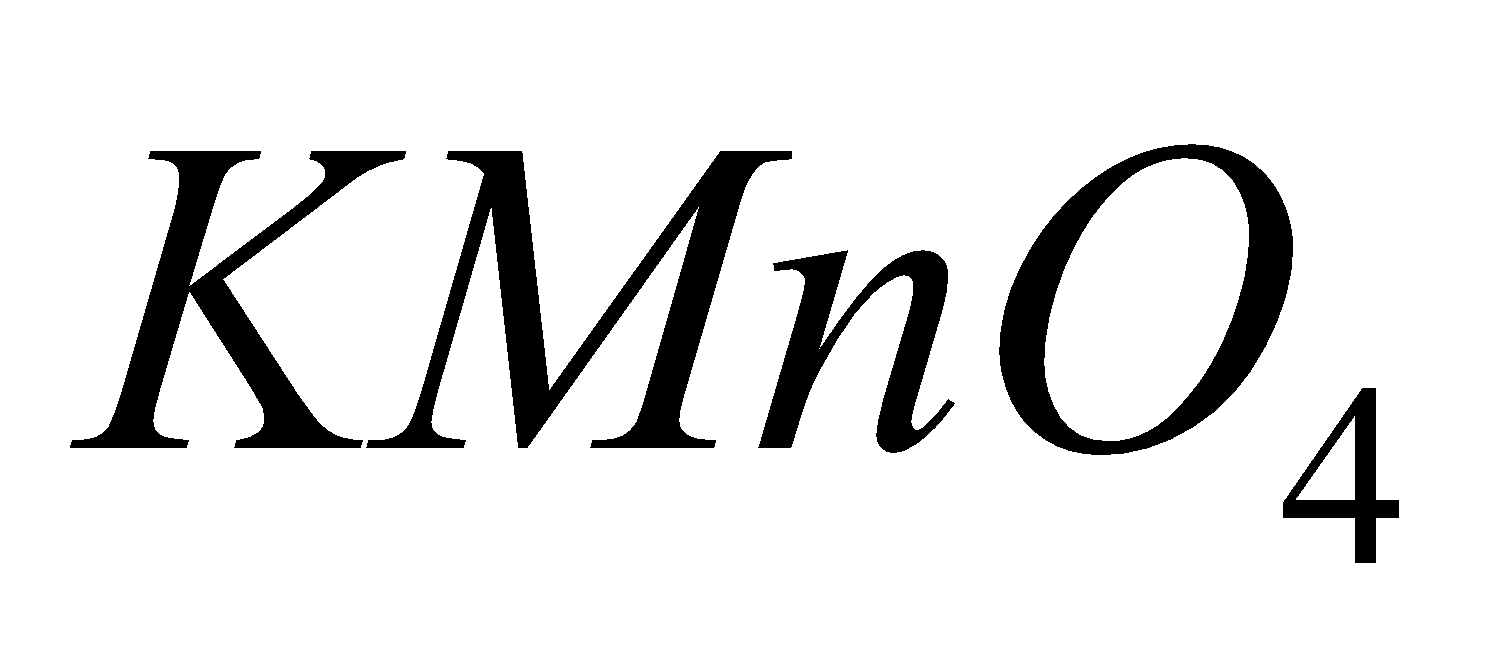

 + Mn
+ Mn  +
+ 
Pot. permanganate Pot. Manganate
NITRITE
COMMON ALUM
NITRIC ACID
4
 4
4