s-BLOCK ELEMENTS - ALKALI METALS
ELEMENTS OF GROUP 1
Li - Lithium Na - Sodium K - Potassium
Rb - Rubidium Cs - Caesium Fr - Francium
These elements are known as alkali metals.
Lithium is known as a bridge element and was discovered by Arfwedson.
Sodium and potassium were discovered by Davy, rubidium and caesium by Bunsen and Kirchhoff while francium by Perey.
These do not occur in the native state (i.e.,do not occur free in nature).
GENERAL CHARACTERISTICS
Physical properties of alkali metals are:-
ELECTRONIC CONFIGURATION
These are s-block elements and have one electron in the valence shell in
s-orbital. In general their electronic configuration may be represented as [noble gas ] ns1 where ‘n’ represents the valence shell.
s-orbital. In general their electronic configuration may be represented as [noble gas ] ns1 where ‘n’ represents the valence shell.
Element
|
Atomic no.
|
Electronic configuration
|
Valence shell configuration
|
Li
|
3
|
[He] 2 s1
|
2 s1
|
Na
|
11
|
[Ne] 3 s1
|
3 s1
|
K
|
19
|
[Ar] 4 s1
|
4 s1
|
Rb
|
37
|
[Kr] 5 s1
|
5 s1
|
Cs
|
55
|
[Xe] 6 s1
|
6 s1
|
Fr
|
87
|
[Rn] 7 s1
|
7 s1
|
SIZE OF THE ATOMS - ATOMIC RADII
- The alkali metals atoms have the largest atomic radii in their respective periods.
- Atomic radii increases as we move down the group from Li to Cs due to the addition of a new shell at each step.
SIZE OF THE ION - IONIC RADII
- The ions of the alkali metals are much smaller than their corresponding atomic radii due to lesser number of shells and contractive effect of the increased nuclear charge.
- The ionic radii like atomic radii of all these alkali metal ions goes on increasing on moving down the group because of the same reason.
DENSITY
- These are light metals having low densities. Lithium is the lightest known metal.
- On moving down the group, both the atomic size and atomic mass increases and since the increase in latter is not compensated by increase in former,consequently density increases from Li to Cs.
- The density of potassium is lesser than that of sodium because of the abnormal increase in size on moving from Na to K.
MELTING AND BOILING POINTS
- The melting and boiling points of alkali metals are quite low and decreases down the group due to weakening of metallic bond.
- Fr is a liquid at room temperature.
SOFTNESS
These are soft,malleable and ductile solids which can be cut with knife. They possess metallic lustre when freshly cut due to oscillation of electrons.
ATOMIC VOLUME
Atomic volume of alkali metals is the highest in each period and goes on increasing down the group
Element
|
Li
|
Na
|
K
|
Rb
|
Cs
|
Gram atomic volume in cm3
|
13
|
24
|
46
|
56
|
71
|
IONISATION ENERGY
- The first ionisation energy of alkali metals is the lowest amongst the elements in their respective periods and decreases on moving down the group.
Element
|
Li
|
Na
|
K
|
Rb
|
Cs
|
Fr
|
IE, (kJ mol–1)
|
520
|
496
|
419
|
403
|
376
|
----
|
- The second ionisation energies of all the alkali metals are very large because on releasing an electron from the elements, the resulting ions acquire noble gas (stable) configurations.
ELECTROPOSITIVE CHARACTER
Because of their low ionisation energies, alkali metals are strongly electropositive or metallic in nature and this character increases from Li to Cs.
CRYSTAL STRUCTURE
All alkali metals possess body centred cubic structures with coordination number 8
OXIDATION STATE
- The alkali metal atoms show only +1 oxidation state, because their unipositive ions have the stable gas electronic configuration in the valence shell.
- Since the alkali metal ions have noble gas configuration with no unpaired electrons, they are diamagnetic and colourless but their permanganates and dichromates compounds are coloured.
HYDRATION OF IONS
- All alkali metal salts are ionic (except Lithium) and soluble in water due to the fact that cations get hydrated by water molecules. The degree of hydration depends upon the size of the cation. Smaller the size of a cation, greater is its hydration energy.
Relative ionic radii :
Relative ionic radii in water or relative degree of hydration :
- The alkali metal ions exist as hydrated ions
in the aqueous solution.
- Since the degree of hydration of
decreases as we go down the group, the hydration energy of alkali metal ions decreases from
FLAME COLOURATION
- All alkali metals and their salts impart characteristic colours to the flame because of the bonding of the outermost electron.The outer electrons of these atoms are excited to higher energy levels. On returning to the original state they give out visible light of characteristic wavelength. This gives a characteristic colour to the flame.
- On moving down the group, the ionisation energy goes on decreasing and hence the energy or the frequency of emitted light goes on increasing in the order Li < Na < K < Rb < Cs. As a result, the colour shows following trend-
Li
|
Na
|
K
|
Rb
|
Cs
|
crimson
|
golden
|
pale
|
purple
|
sky blue
|
red
|
yellow
|
violet
|
(violet)
|
PHOTOELECTRIC EFFECT
Due to low I.E., alkali metals especially K and Cs show photoelectric effect (i.e. eject electrons when exposed to light) and hence are used in photoelectric cells.
ELECTRICAL CONDUCTIVITY
Due to the presence of loosely held valence electrons which are free to move throughout the metal structure, the alkali metals are good conductors of heat and electricity. Electrical conductivity increases from top to bottom in the order 
REDUCING CHARACTER
- All the alkali metals are good reducing agents
and it is due to their low ionisation energies.
Their reducing character, follows the order - Among the alkali metals Li has the highest negative electrode potential, which depends upon its (i) heat of vaporisation (ii) ionisation energy and (iii) heat of hydration and hence Li is the strongest reducing agent.
Elements
|
Li
|
Na
|
K
|
Rb
|
Cs
|
Fr
|
(V)at at 298 K
|
–3.05
|
–2.71
|
–2.93
|
–2.99
|
–2.99
|
—
|
CHEMICAL PROPERTIES
ALKALI METALS FORM IONIC COMPOUNDS
(Lithium can form covalent compounds because of its high ionisation energy) and others form ionic compounds because of their large atomic size and low I.E.
ALKALI METALS ARE VERY REACTIVE
Due to low I.E. and high electropositive character the alkali metals are chemically very reactive.
ACTION OF AIR
On exposure to moist air, their surface is tarnished due to the formation of their oxides, hydroxides and carbonates at the surface.
Hence they are kept under inert liquid kerosene oil but lithium is kept wrapped in paraffin wax because it floats on the surface of kerosene oil due to its very low density.
ACTION OF OXYGEN
- All the alkali metals when heated with oxygen form different types of oxides for example, lithium forms lithium oxide, sodium forms sodium peroxide
, while K, Rb and Cs form their respective superoxides (
where M=K, Rb or Cs). The increasing stability of peroxides and superoxides of alkali metals from Li to Cs is due to stabilisation of larger anions by larger cations through lattice energy.
- Superoxides are coloured and paramagnetic as these possess three electron bond
where one unpaired electron is present.Sodium peroxide acquires yellow colour due to the presence of traces of superoxide as an impurity.is
orange,
is brown and
is orange in colour.
- All oxides, peroxides and superoxides are basic in nature.
- The solubility and basic strength of oxides increase in the order
- The stability of peroxides and superoxides increases in the order
ACTION WITH WATER AND OTHER COMPOUNDS CONTAINING ACIDIC HYDROGEN
- All the alkali metals readily react with water evolving hydrogen.
The reactivity with water increases on descending the group from Li to as
Li < Na < K < Rb < Cs due to increase in electropositive character in the same order.
Li < Na < K < Rb < Cs due to increase in electropositive character in the same order.
- Alkali metals also react with alcohols and acetylene and liberate
ACTION OF HYDROGEN
Alkali metals combine with hydrogen to form ionic hydrides M+H-
The reactivity of alkali metals towards hydrogen decreases as we move down the group i.e. Li > Na > K > Rb > Cs, due to the decreasing lattice energy of these hydrides with the increasing size of the metal cation. Thus the stability of hydrides follows the order
LiH > NaH > KH > RbH > CsH
REACTION WITH HALOGENS
- Alkali metals combine readily with halogens to form ionic halides
[where M= Li,Na, K etc. and X = F,Cl, Br,I]
- The reactivity of alkali metals towards a particular halogen increases in the order :
Li < Na < K < Rb < Cs
while that of halogen towards a particular alkali metal decreases in the order :
- All alkali halides except LiF are freely soluble in water (LiF is soluble in non-polar solvents. Since it has strong covalent bond.)
- The power of the cation to polarise the anion is known as the polarising power while the tendency of the anion to get polarised is known as its polarisability. The polarising power of cation and polarisability of anion depends on the following factors (which are collectively referred to as Fajan’s rules)
- Size of the cation - Smaller the size of cation greater is its polarising power. So LiCl is more covalent than KCl.
- Size of the anion - Bigger the anion, larger is its polarisability. Hence the covalent character of lithium halides is in the order -
LiI > LiBr > LiCl > LiF
- Charge of the ion and electronic configuration - Larger the charge on the cation, greater is its polarising power
Thus the covalent character of various halides is in the order
when two cations have same charge and size, the one having 18 electrons in their outermost shell will have larger polarising power than a cation having 8 electrons in the outermost shell. For example CuCl is more covalent than NaCl.
Above rules help to predict the ionic /covalent character of metal halides.
MELTING POINTS OF ALKALI METAL HALIDES
- For the same alkali metal, the melting points decrease in the order with the increase in the size of halides ion. Fluorides > chlorides > bromides > iodides
- For the same halide ion, melting points decreases with the increasing size of the metal but lithium halides being covalent have lower melting point than corresponding sodium halides.
REACTION WITH NITROGEN
Only lithium reacts with nitrogen and forms lithium nitride ( )
)
REACTION WITH SULPHUR AND PHOSPHORUS
Alkali metals react with sulphur and phosphorus on heating and form respective sulphides and phosphides.
SOLUBILITY IN LIQUID AMMONIA
All alkali metals dissolve in liquid ammonia giving deep blue solution, which has some characteristic properties given below due to formation of ammoniated metal cations and ammoniated electrons in the solution.
- Colour - The blue colour is due to the excitation of ammoniated electron to higher energy levels and the absorption of photons occurs in the red region of the spectrum.Thus the solution appears blue.But at very high concentration the solution attains the colour like that of metallic copper.
- Conductivity - It is highly conducting because of the presence of ammoniated electrons and ammoniated cations.However, on cooling,the conductivity increases further.
- Paramagnetism - It is paramagnetic due to the presence of an unpaired electrons and ammoniated cations.However the paramagnetism decreases with increasing concentration due to the association of ammoniated electrons to yield diamagnetic species containing electron pairs.
- Reducing property - Due to the presence of ammoniated electrons, solution is a very powerful reducing agent and used in organic chemistry under the name Birch reduction.
COMPLEX FORMATION
Alkali metals have a weak tendency to form complexes but polydentate ligands such as crown ethers and cryptands form highly stable complexes collectively called as Wrap Around Complexes. Cryptands are macrocyclic molecules with N and O atoms and their complexes are called cryptates. The name cryptate came from the fact that metal ion is hidden in the structure.
NATURE OF HYDROXIDES
Alkali metals hydroxides are very strong bases, highly soluble in water and are not decomposed on heating.However, LiOH decomposes on heating to give because latter is more stable than former.
Their basic strength increases from LiOH to CsOH due to a corresponding decresae in the I.E., of the metal in a group,i.e., the order:-
LiOH < NaOH < KOH < RbOH < CsOH
NATURE OF CARBONATES AND BICARBONATES
is unstable towards heat and decomposes to give
The thermal stability of carbonates increases with the increasing basic strength of metal hydroxides on moving down the group.Thus the order is
- The bicarbonates of all the alkali metals are known. All the bicarbonates (except which exits in solution) exist as solids and on heating form carbonates.
- The solubility of the carbonates and bicarbonates increases on moving down the group due to lower lattice energies. Thus, order is
NATURE OF NITRATES
On heating decomposes to give 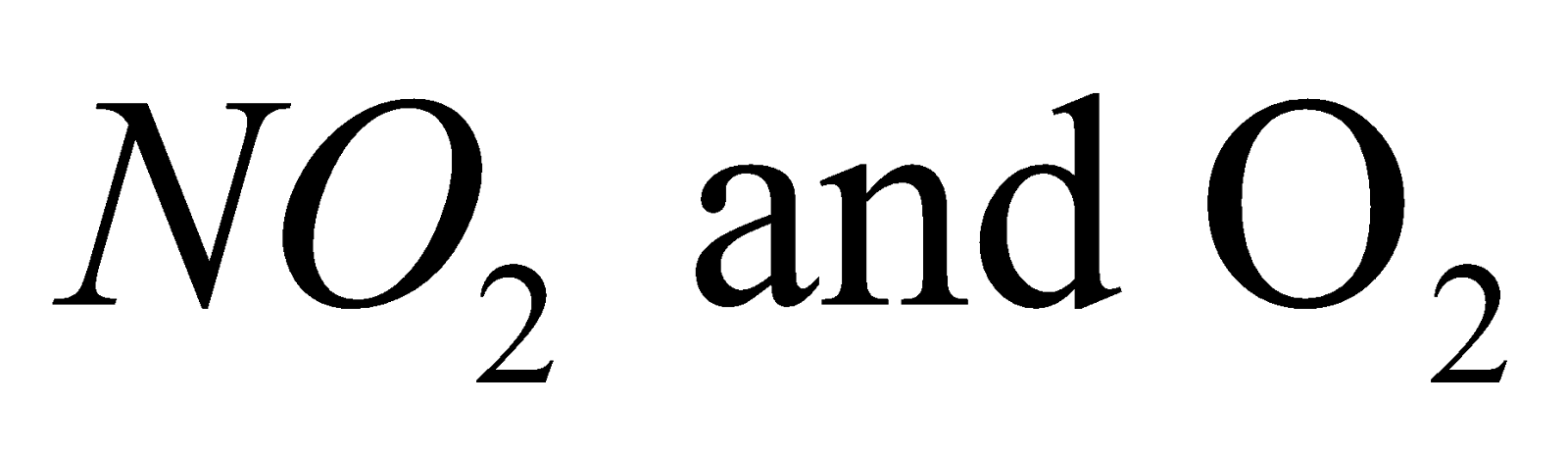 while the nitrates of the other alkali metals decompose on heating to form nitrites and.
while the nitrates of the other alkali metals decompose on heating to form nitrites and.
All nitrates are soluble in water.
NATURE OF SULPHATES
- Li2SO4 is insoluble in water whereas other sulphates i.e, Na2SO4, K2SO4 are soluble in water.
- Lithium sulphate does not form alums and is also not amorphous with other sulphates.
ANOMALOUS BEHAVIOUR OF LITHIUM
Lithium, the first member of the alkali metal family shows an anomalous behaviour because of the following main reasons:-
- It has the smallest size in the group
- It has very high ionization energy and highest electronegativity in the group.
- It has no vacant d-orbital in the valence shell.
As a result, it differs from the other member of the alkali metal family in following respects:
- Lithium is harder than other alkali metals, due to strong metallic bond.
- Lithium combines with
to form lithium monoxide,
, whereas other alkali metals form Peroxides
, and superoxides
.
- Lithium, unlike the other alkali metals, reacts with nitrogen to form the nitride.
- LiOH is a weak base and decomposes to give the corresponding oxide while the hydroxides of alkali metals are stable to heat and sublime as such.
, LiF and lithium phosphate are insoluble in water while the corresponding salts of other alkali metals are soluble in water.
- LiH is the stablest among all the alkali metal hydrides.
decomposes on heating to evolve
whereas other alkali metal carbonates do not.
- Lithium nitrate on heating evolves O2 and NO2 and forms Li2O while other alkali metal nitrates on heating evolve and form their respective nitrites.
- Lithium when heated with ammonia forms lithium imide
while other alkali metals form amides of the general formula (
where M=Na,K, Rb and S).
- Only lithium combines directly with carbon to form lithium carbide,
, while other alkali metals react with ethyne to form the corresponding metal carbides.
DIAGONAL RELATIONSHIP
Lithium shows diagonal relationship with magnesium, the element of group 2 and this resemblance is due to polarising power, i.e,  is similar for both of these elements.
is similar for both of these elements.
Lithium resembles magnesium in the following respects:
- The atomic radius of Lithium is
while that of magnesium is
- The ionic radius of which is very close to that of Mg2+ ion (0.65Å).
- Lithium (1.0) and magnesium (1.2) have almost similar electronegativities.
- Both Li and Mg are hard metals.
- LiF is partially soluble in water like
.
- Both decompose water only on heating.
- Alkyls of lithium and magnesium are soluble in organic solvents.
- Both combine with
to form monoxides,e.g.,
and MgO.
- Both LiOH and
are weak bases.
- Both LiCl and
are predominantly covalent.
- Both Li and Mg combine with
to form their respective nitrides,
and
.
- The hydroxides and carbonates of both Li and Mg decompose on heating and form their respective oxides.
- Both lithium and magnesium nitrates on heating evolve
and
leaving behind their oxides.
Fire caused by burning of alkali metals is extinguished by sprinkling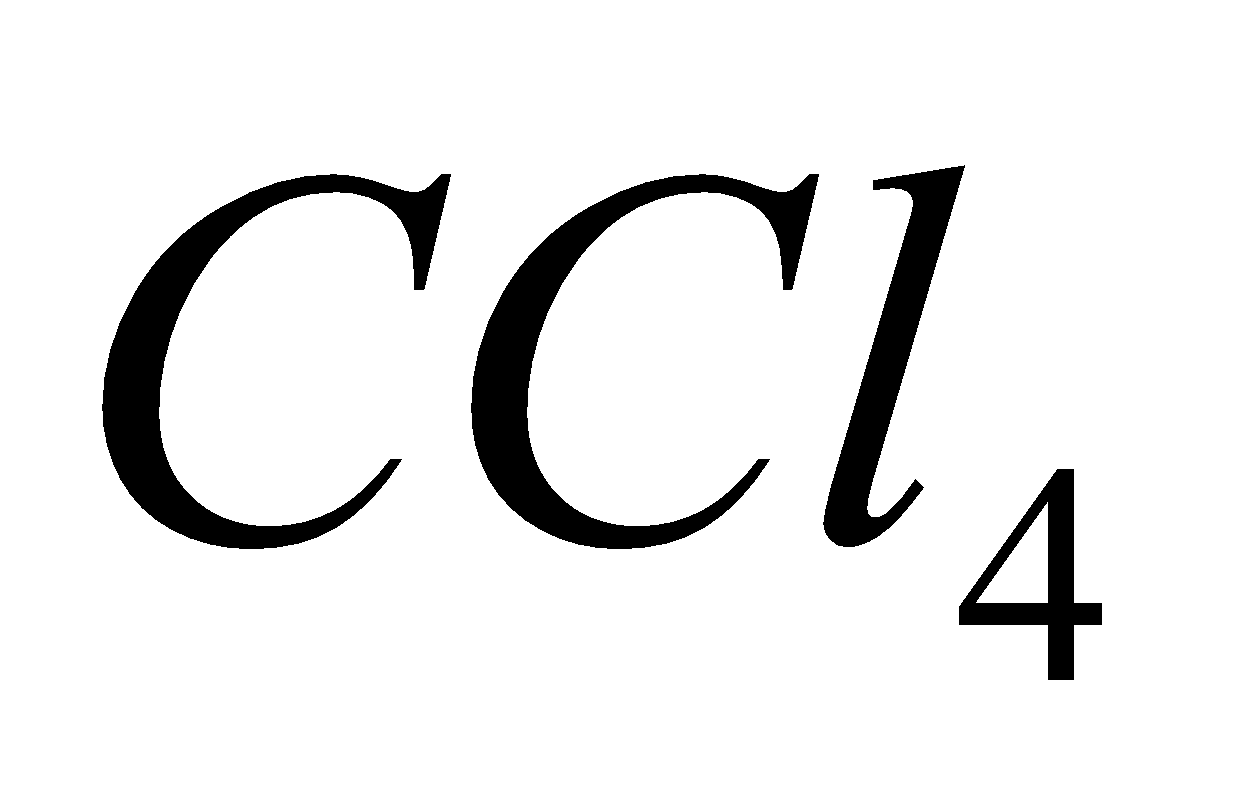 .
.
A mixture of  and dil.HCl is commercially called Oxone and is used for bleaching delicate fibres.
and dil.HCl is commercially called Oxone and is used for bleaching delicate fibres.
METALLURGY OF SODIUM
OCCURRENCE AND MINERALS
- Sodium does not occur in the free state because of its high reactivity.
- Important minerals of sodium are -
- Common salt or rock salt,NaCl
- Chile saltpetre,
- Sodium carbonate,
- Sodium sulphate or Glauber’s salt Na2SO4. 10 H2O
- Cryolite,
- Borax,
EXTRACTION OF SODIUM
- Sodium metal is extracted by electrolysis of fused NaCl containing a little and KF at 873 K. This process is known as Down process.
- Reactions during electrolysis
- Difficulties during the process
- Sodium cannot be extracted from aqueous NaCl because the metal liberated at the cathode reacts with to form metal hydroxide and
.
- NaCl melts at 800º C and it is difficult to attain and maintain this high temperature.
- Molten Na forms a metallic fog (colloidal solution ) with fused NaCl.
Above difficulties were removed by adding  and KF to fused NaCl which themselves do not undergo decomposition at the voltage employed and lower the melting point of NaCl to about. The electrodes are separated by a wire gauze to prevent the reaction between Na and
and KF to fused NaCl which themselves do not undergo decomposition at the voltage employed and lower the melting point of NaCl to about. The electrodes are separated by a wire gauze to prevent the reaction between Na and
METALLURGY OF POTASSIUM
OCCURRENCE AND MINERALS
- Potassium also does not occur in free state.
- Important minerals of potassium are
- Sylvine, KCl
- Carnallite,
- Feldspar,
- Kainite,
EXTRACTION OF POTASSIUM
Potassium is not obtained by the electrolysis of fused KCl because K has lower boiling point (1039 K) than the melting point of KCl (1063K) and hence it get vaporises. Therefore, K metal is extracted by the following methods :-
- By the electrolysis of fused KOH - The reaction involved are
- Modern method - By the reduction of molten KCl with metallic sodium in stainless steel vessel at 1120-1150 K.
COMPOUNDS OF SODIUM
SODIUM CHLORIDE, COMMON SALT OR TABLE SALT, NaCl
- It is obtained by evaporation of sea water in sun but due to presence of impurities like
it is deliquescent It is purified by passing HCl gas through the impure saturated solution of NaCl and due to common ion effect, pure NaCl gets precipitated.
- 28% NaCl solution is called Brine.
SODIUM HYDROXIDE, CAUSTIC SODA, NaOH
PREPARATION
- Causticizing process ( Gossage process) - A 10% solution of
is treated with milk of lime,
.
- Electrolytic process - In this process a concentrated solution of sodium chloride is electrolysed where
is evolved at the anode and at the cathode. However gas
reacts with NaOH forming NaCl and sodium hypochlorite.
Mercury cathode process (Castner - Kellner cell)
This process is used to avoid reaction between NaOH and .NaOH is obtained by the electrolysis of (aqueous) solution of brine. The cell has three compartments and involves following reactions :-
In outer compartment -
Anode - Graphite rods
Cathode - Mercury
Electrolyte -Brine solution
Reaction - At Anode :
At Cathode : 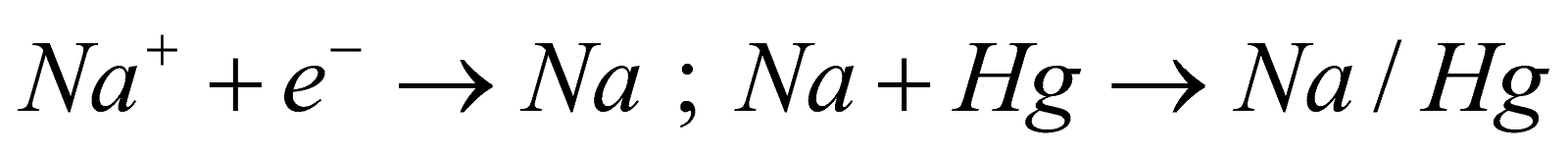
In central compartment -
Anode - Mercury
Cathode - Iron rods
Electrolyte - dil. solution of NaOH
Reaction - At Anode :
At Cathode : 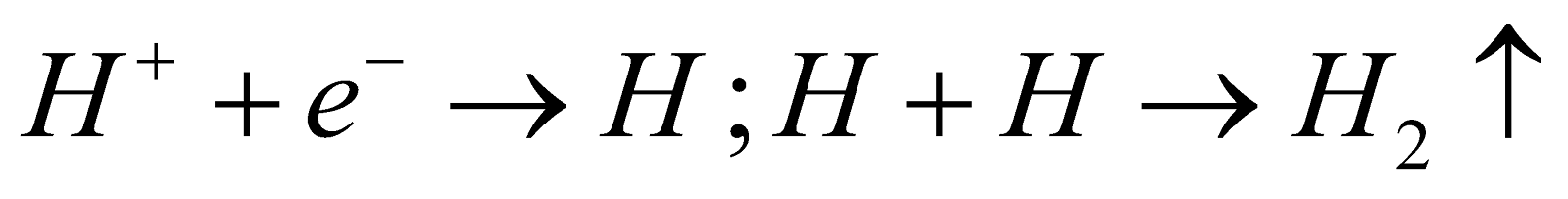
- Lowing's Process
- Pure Sodium Hydroxide
The filtration on evaporation give pure NaOH
PROPERTIES
- It is a hygroscopic, deliquescent white solid, absorbs CO2 and moisture from the atmosphere.
- Reaction with salts:- It reacts with metallic salts to form hydroxides out of which some are unstable and decompose to insoluble oxides,
- Formation of insoluble hydroxides, e.g.
- Formation of unstable hydroxides, e.g.
- Formation of insoluble hydroxides which dissolve in excess of NaOH e.g. Zn, Al, Sb, Pb, Sn and As.
- Formation of ammonia from ammonium salts :-
- Reaction with halogens:-
- Reaction with metals :- Less electropositive metals like Zn, Al and Sn etc. give H2 gas with NaOH.
- Reaction with sand:-
- Reaction with CO :-
- Reaction with non-metals e.g.with P, Si, S, F, etc.
- It breaks down the proteins of the skin flesh to a pasty mass and hence it is commonly known as caustic soda.
SODIUM CARBONATE OR WASHING SODA  :
:
PREPARATION
- Solvay or ammonia - soda process :- In this process,NaCl (brine), ammonia and
are taken as raw materials. The involving reactions are
- Electrolytic Process :- In this Nelson cell is used for the manufacture of NaOH, CO2 under pressure is blown with steam
- Leblance Process :-This is now an absolute method.
PROPERTIES
- Sodium Carbonate crystallizes from water as decahydrate which efflorescence on exposure to dry air forming monohydrate which on heating change to anhydrous salt (soda-ash).
- On hydrolysis it forms an alkaline solution
- Aqueous sodium carbonate solution react with CO2 gas and forms sodium bicarbonate.
- It is used as fusion mixture
SODIUM BICARBONATE, BAKING SODA, NaHCO3
PREPARATION
It is obtained as an intermediate product in Solvay ammonia process.
PROPERTIES
- Heating effect :- It gives
- In aqueous medium it is alkaline due to hydrolysis:
- It is used as a constituent of baking powder and in medicine to remove acidity of the stomach (as antacid).
- It is present in Selidlitz powder.
- Baking powder is a mixture of starch, sodium bicarbonate and potassium hydrogen tartarate.
- Fire extinguishers contain
is called Glauber’s salt, anhydrous
is called salt cake,
is called chile salt-petre,
is called nitre cake, mixture
of and dil. HCl is called oxone.
- When common salt is fused with a little
, 5% to 10%and some sugar, it acquires a dark purple colour and has a characteristic saline taste. It is used in medicine and is useful for digestion.It is called kala namak or black salt or sulemani namak.
COMPOUNDS OF POTASSIUM
POTASSIUM HYDROXIDE,CAUSTIC POTASH, KOH
PREPARATION
- It is prepared in a cell similar to that used for NaOH. In this cell electrolysis of an aqueous solution of KCl takes place.
- It is also prepared by the action of soda lime
on potassium carbonate.
POTASSIUM CARBONATE, POTASH, PEARL ASH, K2CO3
PREPARATION
It is prepared by following two methods-
- By Leblanc process
- By Precht process (magnesia process)
POTASSIUM CYANIDE, KCN:
PREPARATION
- By heating potassium ferrocyanide with metallic potassium
- It is used in electroplating and due to the formation of soluble complexes with gold and silver, it is used in extraction of these metals.
POTASSIUM CHLORATE, KCLO
PREPARATION
- By passing
through boiling concentrated KOH solution.
- By the action of KCl on (obtained by electrolysis of NaCl at 345-350K).
PROPERTIES
It used as an oxidising agent and in the laboratory and preparation of  .
.










