VOLUMETRIC ANALYSIS
VOLUMETRIC METHODS
Volumetric or titrimetric analysis are quantitative analytical techniques which employ a titration in comparing an unknown with a standard. In a titration, a volume of a standardized solution containing a known concentration of reactant ‘A’ is added incrementally to a sample containing an unknown concentration of reactant ‘B’ till reactant ‘B’ is just consumed (stoichiometric completion). This is known as the equivalence point.
At this point we have N1V1 = N2V2.
INDICATOR
A compound added to the reacting solutions that undergo an abrupt change in a physical property usually a colour .
TYPES OF INDICATORS
- Internal - They are added to the reacting solutions
- External - Electrochemical devices such as pH meters
END POINT
The point at which a titration is stopped i.e. equivalence point.
STANDARD SOLUTION
The solution of known concentration of an acid, base or salt.
EXAMPLES OF VOLUMETRIC METHODS
ACIDIMETRY AND ALKALIMETRY
Sodium Carbonate - Hydrochloric acid Titration
Reaction : 
Indicator : Methyl orange
End Point : Appearance of light pink colour
In presence of phenolphthalein the reaction between Na2CO3 and HCl is half complete
In presence of phenolphthalein the reaction between Na2CO3 and HCl is half complete
PRECIPITATION TITRATIONS
- AgNO3 – NH4CNS OR KCNS TITRATION (VOLHARD'S METHOD)
Reaction :
Slight excess of KCNS or NH4CNS gives blood red tinge with ferric salt.
Indicator : Ferric alum solution
End Point : Appearance of blood red tinge
- AgNO3 - NaCl TITRATION (MOHR'S METHOD)
Reaction : 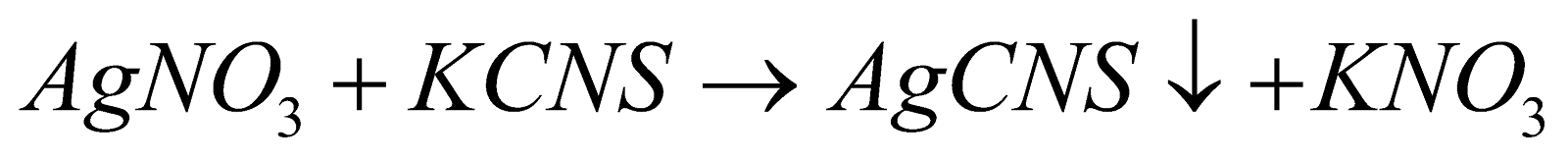
Slight excess of AgNO3 gives a brick red ppt. with K2CrO4
Indicator : Potassium Chromate solution
End Point : Appearance of brick red precipitate
REDOX TITRATIONS
OXALIC ACID - POTASSIUM PERMANGANATE TITRATION
Reaction :
Indicator : KMnO4 is self indicator
End Point : Appearance of light pink colour
FERROUS AMM. SULPHATE - KMnO4 TITRATION
Reaction :
Indicator : KMnO4 is self indicator
End Point : Appearance of light pink colour
FERROUS AMM. SULPHATE - K2Cr2O7 TITRATION
Reaction :
Potassium ferricyanide gives blue coloured ppt. with ferrous ions
Indicator : Potassium ferricyanide (External) or Diphenylamine (internal).
End point : No green colour when K3[Fe(CN)6] is used.
Bluish violet or purple colour.
When  is used.
is used.
I2-Na2S2O3.5H2O TITRATION
This is Iodometry titration. Since standard solution of iodine is used.
Reaction : 
Indicator : Freshly prepared starch solution.
End Point : Disappearance of blue colour.
Iodine is dissolved in KI solution and forms soluble KI3
KI + I2 ⇌ KI3
Equilibrium shifts to left hand side when iodine is required.
As2O3-I2 TITRATION (IODOMETRY TITRATION)
Reaction : 
Solution of arsenious oxide is prepared in NaOH.
HI is removed by adding NaHCO3 to make reaction irreversible.
Indicator : Freshly prepared starch solution.
End point : Disappearance of blue colour.
IODOMETRY
Iodine is liberated during chemical reactions.
Reaction :
Indicator : Freshly prepared starch solution
End point : Disappearance of blue colour.
K2Cr2O7-Na2S2O3.5H2O TITRATION (IODOMETRY)
Reaction :
Indicator : Freshly prepared starch solution.
End point : Disappearance of blue colour.
COMPLEXATION REACTIONS
HARDWATER-EDTA TITRATION
Reaction :  stable complex
stable complex
Indicator : Eriochrome black-T (Alcoholic solution)
End point :  of water + Eriochrome black-T
of water + Eriochrome black-T
Thus wine red colour changes to blue at end point
- Acid base titration involves proton (H+) transfer reaction between analyte and titrant.
- Precipitation reactions, ions in solution combine to form an insoluble precipitate.
- Complexation reactions, analyte and titrant combine to form a very stable complex ion (held together by coordinate bonds)
- Redox reaction, electrons transfer between analyte and titrant.
PRIMARY STANDARD
It is a reagent that is pure enough to be weighed out and used directly to provide a known number of moles eg. Na2CO3, FeSO4 (NH4)2SO4.6H2O, etc.
STANDARD SOLUTION
A solution whose concentration is known. The concentration is generally expressed in terms of normality.
EQUIVALENT MASS
It is the mass in gram which is chemically equivalent to 1.008 g of hydrogen, 8g of oxygen or 35.46 g of chlorine.
Equivalent mass of an acid= 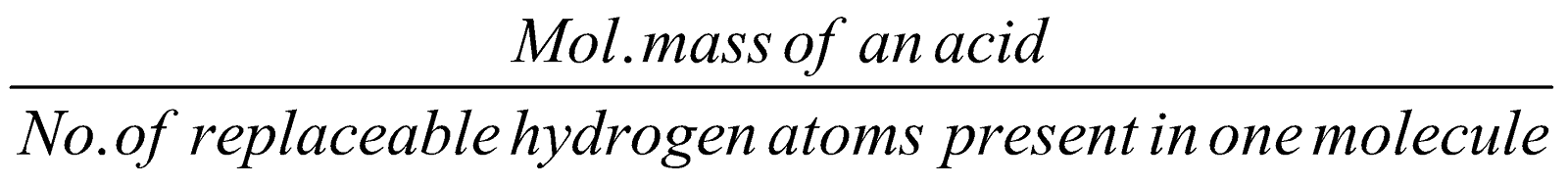
Equivalent mass of a base = 
Equivalent weights of oxidising and reducing agents. On the basis of the change in O.N. of an element in the oxidising or reducing agent.
Eq. wt. = 
Thus in acidified KMnO4, Mn in ion is reduced to Mn2+, the change in O.N. is 5.
Hence equivalent weight of KMnO4 in acidified solution
Eq. wt. = 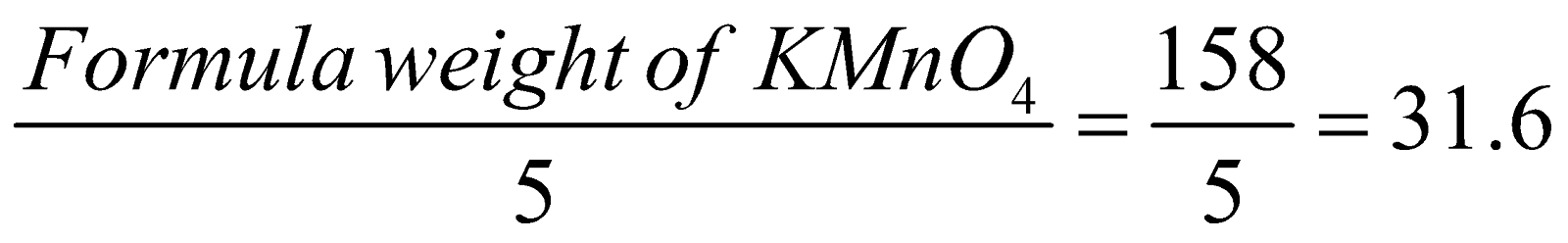
In alkaline medium Mn in ion is reduced to MnO2, the change in O.N. is 3.
Hence equivalent weight of KMnO4 in alkaline medium.
Eq. wt. = 
Fe in FeSO4 is oxidised to Fe2(SO4)3, a change in O.N. is 1. Hence equivalent weight of FeSO4 is
Eq. Wt. = 
In acidified solution Cr in K2Cr2O7, is reduced to Cr3+, the total change in O.N. is 6.
Hence equivalent weight of K2Cr2O7 is
Eq. wt. = 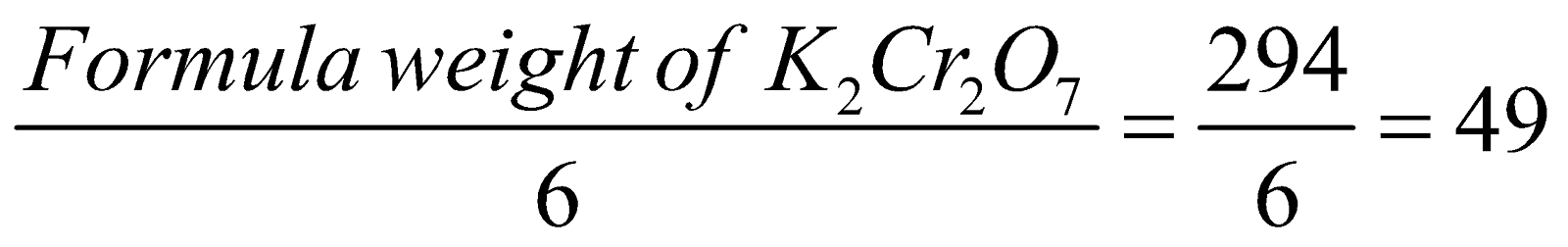
Equivalent mass of a salt =
STRENGTH OF THE SOLUTION
It is expressed in grams per litre and related to normality and equivalent weight as,
Strength = Normality × equivalent weight.
RELATION BETWEEN NORMALITY AND MOLARITY
Normality = n × Molarity
where n = number of equivalents in 1 mole










