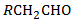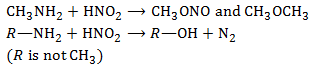The one subject in NEET which is candidates who can easily attain good marks is Chemistry. That's the reason, often, one doesn’t pay notice and choose to compromise it. But if one wants to rank above others, the tip is to be thorough with NEET chemistry concepts. The understanding of reactions and definite basic understanding is what requires major attention in Chemistry but once done it only gets simpler from there. The main focus on the to-do list should be on getting a hang of the NCERT syllabus of NEET chemistry..
Q2.Primary alcohols can be obtained from the reaction of the RMgX with:
Solution
HCHO
HCHO
Q4. Which of the following reactions does not yield an ether?
Solution
2° alkyl halides tend to undergo elimination. Thus bromocyclopentane on treatment with sodium ethoxide gives cyclopentane rather than cyclophenyl ethyl ether
2° alkyl halides tend to undergo elimination. Thus bromocyclopentane on treatment with sodium ethoxide gives cyclopentane rather than cyclophenyl ethyl ether
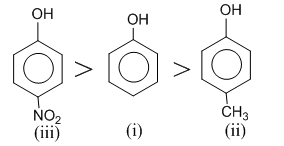
Q5.Correct acidic order of the following compounds is
Solution
Presence of electron withdrawing group such as etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxide ion while presence of electron releasing groups such as
etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxide ion while presence of electron releasing groups such as destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compound is
destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compound is
Presence of electron withdrawing group such as
 etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxide ion while presence of electron releasing groups such as
etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxide ion while presence of electron releasing groups such as destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compound is
destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compound is
Q7.Which compound will have highest boiling point?
Solution
The boiling point of alcohols is higher than the boiling points of corresponding alkanes and aldehydes due to H-bonding. As the molecule mass increases, boiling point increases. Thus, has the higher boiling point among the given.
has the higher boiling point among the given.
The boiling point of alcohols is higher than the boiling points of corresponding alkanes and aldehydes due to H-bonding. As the molecule mass increases, boiling point increases. Thus,
 has the higher boiling point among the given.
has the higher boiling point among the given.
Solution
−OH group is an activating group, hence increase electron density on o-and p-position in benzene ring. Thus, phenol very easily undergoes nitration (electrophilic substitution and give trinitrophenol, i.e., picric acid).
−OH group is an activating group, hence increase electron density on o-and p-position in benzene ring. Thus, phenol very easily undergoes nitration (electrophilic substitution and give trinitrophenol, i.e., picric acid).
Q9.Which of the following reagents may be used to distinguish between phenol and benzoic acid?
Solution
The President allocates the portfolios to the central ministers on the advice of the Prime Minister
The President allocates the portfolios to the central ministers on the advice of the Prime Minister
Q10. Alcohols may behave as:
Solution
Alcohols are neutral as they do not influence the pH. Due to O—H bond, they possess Bronsted acid nature showing cleavage of O—H bond. Also due to the presence of lone pair of electron on oxygen atom, they act as Lewis base. The reactivity order is based on +IE of alkyl groups. Lewis base order :
Alcohols are neutral as they do not influence the pH. Due to O—H bond, they possess Bronsted acid nature showing cleavage of O—H bond. Also due to the presence of lone pair of electron on oxygen atom, they act as Lewis base. The reactivity order is based on +IE of alkyl groups. Lewis base order :
3 ͦ >2 ͦ >1 ͦ Bronsted acid order : 1 ͦ >2 ͦ >3 ͦ