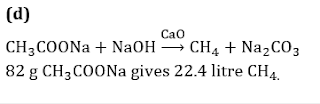Hydrocarbons Quiz-7
Dear Readers,
The one subject in NEET which is candidates who can easily attain good marks is Chemistry. That's the reason, often, one doesn’t pay notice and choose to compromise it. But if one wants to rank above others, the tip is to be thorough with NEET chemistry concepts. The understanding of reactions and definite basic understanding is what requires major attention in Chemistry but once done it only gets simpler from there. The main focus on the to-do list should be on getting a hang of the NCERT syllabus of NEET chemistry..
Q1. Presence of a nitro group in a benzene ring
Solution
(d) -NO2 group withdraw electron from the ring shows-M effect makes ring electron deficient, thus deactivates ring for electrophilic substitution.
(d) -NO2 group withdraw electron from the ring shows-M effect makes ring electron deficient, thus deactivates ring for electrophilic substitution.
Q4. Reaction of trans-2-phenyl-1-bromocyclopentane on reaction with alcoholic KOH produces:
Solution
(c) 1-phenylcyclopentene
(c) 1-phenylcyclopentene
Q5.What volume of methane (NTP) is formed from 8.2 g of sodium acetate by fusion with sodalime?
Q6. When a mixture of methane and oxygen is passed through heated molybdenum oxide, the main product formed is
Q7.Two jars A and B are filled with hydrocarbons. Br2 in CCl4 is added to these jars. A does not decolourise the Br2 solution but B decolourises. What are A and B?
Solution
(a)
Br2 solution is decolourized by alkene or alkyne or molecules having unsaturation.
Q8.Which of the following is unsymmetrical alkene?
Solution
(d) e.g., CH3 CH2 CH=CH2 is unsymmetrical. CH3 CH=CHCH3 is symmetrical. Note the positions of carbon atoms on two sides of double bond.
(d) e.g., CH3 CH2 CH=CH2 is unsymmetrical. CH3 CH=CHCH3 is symmetrical. Note the positions of carbon atoms on two sides of double bond.
Q9.When HCI gas is passed through propene in the presence of benzoyl peroxide, it gives:
Solution
(b)
Peroxide effect is noticed only in case of HBr. For HCl follow Markownikoff’s rule.
Q10. The carbon-carbon bond distance in benzene is
Solution
(b)
According to X-ray analysis all carbon-carbon bond distance (1.397Å) are equal in benzene. The bond order of carbon-carbon bond is 1.5 in benzene.
Hence, carbon-carbon bond distance (1.397Å) is less than C-C single bond (1.54Å) and more than C=C double bond(1.33Å).