JEE Advanced is a high level exam that checks your concept by putting various types of questions. The questions are in a different format from JEE mains.m, herein the questions are : single correct multiple choice questions, multiple correct multiple choice questions, statement based questions and comprehension type questions..
Solution
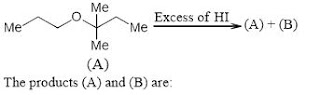
(b) This is an example of intermolecular SN^2Williamson’s reaction
(b) This is an example of intermolecular SN^2Williamson’s reaction
Q2.
An industrial method for preparation of methanol is:
An industrial method for preparation of methanol is:
Solution
(a)
(a)
Q4.
Which of the following is most acidic?
Solution
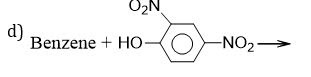
(d) Phenol (hydroxyl benzene) is more acidic than alcohols
(d) Phenol (hydroxyl benzene) is more acidic than alcohols
Q5.Which of the following statements is correct?
i. Glycerol on reaction with oxalic acid at 110°C(383K) and followed by heating and hydrolysis gives formic acid and glycerol
ii. Glycerol on reaction with oxalic acid at 230°C(503K) and followed by heating gives allyl alcohol
iii. Glycerol on oxidation with conc. HNO_3 gives glyceric acid and tartonic acid
iv Glycerol on oxidation with conc. HNO_3 gives glyceric acid
i. Glycerol on reaction with oxalic acid at 110°C(383K) and followed by heating and hydrolysis gives formic acid and glycerol
ii. Glycerol on reaction with oxalic acid at 230°C(503K) and followed by heating gives allyl alcohol
iii. Glycerol on oxidation with conc. HNO_3 gives glyceric acid and tartonic acid
iv Glycerol on oxidation with conc. HNO_3 gives glyceric acid
Solution
(d)
(d)
Q6. Proof is defined as………the percentage by volume of alcohol in ethanol-water mixture
Solution
(a)
(a)
Q10.
Give the decreasing order of reactivity of the following alkyl halides in the Williamson’s reaction
i. (CH_3 )_3 C-CH_2 Br
ii.ClCH_2 CH=CH_2
iii.ClCH_2 CH_2 CH_3
iv.BrCH_2 CH_2 CH_3
Give the decreasing order of reactivity of the following alkyl halides in the Williamson’s reaction
i. (CH_3 )_3 C-CH_2 Br
ii.ClCH_2 CH=CH_2
iii.ClCH_2 CH_2 CH_3
iv.BrCH_2 CH_2 CH_3
Solution
(a) ii>iv>iii>i (allyl chloride>1° RBr>1°RCl>neopentyl bromide. (R-Br) bond is weaker than (R-Cl)bond SN^2 reaction is faster with (R-Br)than with (R-Cl)
(a) ii>iv>iii>i (allyl chloride>1° RBr>1°RCl>neopentyl bromide. (R-Br) bond is weaker than (R-Cl)bond SN^2 reaction is faster with (R-Br)than with (R-Cl)