IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. A compound (60 gm) on analysis gave C = 24 gm, H = 4 gm, and O = 32 gm. Its empirical formula is:
Solution
(b)
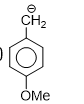
Order of enol content: Aldehyde
(b)
Order of enol content: Aldehyde
Q2.Which of the following is least stable?
Solution
(a) In (b), (c) and (d), carbanion is stabilised by resonance, but in (a) it is not stabilized. Moreover, (+I ) effect of (Me) group destabilizes the carbanion in (a)
(a) In (b), (c) and (d), carbanion is stabilised by resonance, but in (a) it is not stabilized. Moreover, (+I ) effect of (Me) group destabilizes the carbanion in (a)
Q3. Liquid benzene (C6 H6) burns in oxygen according to
2C6H6 (l)+15O_2 (g)→12CO2 (g)+6H2O(g)
How many litres of O_2 at STP are needed for complete combustion of 39 gm of liquid benzene?
Solution


Q4. An SN^2 reaction at an asymmetric C of a compound always gives:
Solution
(d)
SN^2 reaction proceeds with the inversion of configuration; hence, only one stereoisomer is obtained
(d)
SN^2 reaction proceeds with the inversion of configuration; hence, only one stereoisomer is obtained
Q5.The number of isomers of the compound C2 FClBrI is:
Solution
Part D
Part D
Q6. In Liebig's method for the estimation of C and H, if the compound also contains halogens, which of the following is kept near the exit of the combustion tube?
Solution
(c)
Halogens react with Ag to give AgX which reacts with PbCrO4 to precipitate Ag2 CrO4
(c)
Halogens react with Ag to give AgX which reacts with PbCrO4 to precipitate Ag2 CrO4
Q7.The empirical formula of an organic compound is CH2. The mass of 1 mol of it is 42 gm. The molecular formula of the compound is:
Solution


Q8.Which of the following compounds will exhibit cis-trans(geometrical) isomerism?
Solution
(a)
It is due to the restricted rotation around the double bond, so 2-butene shows G.I.
(a)
It is due to the restricted rotation around the double bond, so 2-butene shows G.I.
Q9.

Solution


Q10. An isomer of ethanol is:
Solution
(d)
Ethanol is a functional isomer of dimethyl ether, as both have the same molecular formula
(d)
Ethanol is a functional isomer of dimethyl ether, as both have the same molecular formula