IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. Which of the following compounds will exhibit geometrical isomerism?
Solution


Q2.

Solution
(c) The larger the stability, the smaller the P.E.; henceI>II>III
(c) The larger the stability, the smaller the P.E.; henceI>II>III
Q3. The smallest aldehyde and its next homologue are treated with NH2OH to form oxime. Find out the correct answer out of the following
Solution


Q4. The IUPAC name of the compound with formula C_n H_(2n+2), having the lowest possible molecular mass and capable of showing enantiomerism, is:
Solution
Part D
Part D
Q5.

Solution
Part A
Part A
Q6. The optically active tartaric acid is named as D-(+)-tartaric acid because it has apositive
Solution
(c) The symbol D denotes the relative configuration of (OH) group w.r.t. glyceraldehydes taken as standard. Also, (+) sign refers to optical rotation and is dextrorotatory
(c) The symbol D denotes the relative configuration of (OH) group w.r.t. glyceraldehydes taken as standard. Also, (+) sign refers to optical rotation and is dextrorotatory
Q7.Which of the following compounds has isopropyl group?
Solution
Part D
Part D
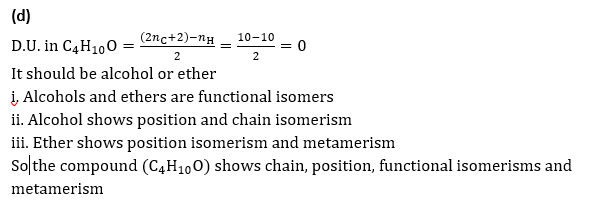
Q8.The type of isomerism exhibited by the compound with formula C4H10O is:
Solution

Q9.Lassigne's test is used for the detection of:
Solution
(a)
It is the test for N, S and halogens. Phosphorous is detected by another method
(a)
It is the test for N, S and halogens. Phosphorous is detected by another method
Q10. 

Solution
(a)
A carboxylic acid is stronger acid than phenol, hence both III and IV are stronger acids than both I and II. Also IV has a methyl group that gives electrons donating inductive effect and decreases the acid strength. Therefore, III is stronger acid than IV. Between I and II, the dominate electron withdrawing inductive effect of chlorine increases acid strength of phenol slightly, hence II is stronger of phenol slightly, hence, II is stronger acid than I. Thus, the overall order is: (a) III>IV>II>I.
(a)
A carboxylic acid is stronger acid than phenol, hence both III and IV are stronger acids than both I and II. Also IV has a methyl group that gives electrons donating inductive effect and decreases the acid strength. Therefore, III is stronger acid than IV. Between I and II, the dominate electron withdrawing inductive effect of chlorine increases acid strength of phenol slightly, hence II is stronger of phenol slightly, hence, II is stronger acid than I. Thus, the overall order is: (a) III>IV>II>I.





