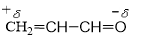IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. Polarisation of electrons in acrolein may be written as:
Solution
(d) O atom is more EN than C atom, so it acquires (-δ) charge and C atom acquires (+δ) charge which is transferred to the last C atom
(d) O atom is more EN than C atom, so it acquires (-δ) charge and C atom acquires (+δ) charge which is transferred to the last C atom
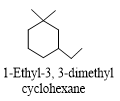
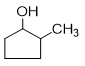
Q2.Which of the following is correctly named?
Solution


Q3. Which of the following is correctly named?
Solution


Q4. How many optically active stereoisomers are possible for butane-2-3-diol?
Solution


Q5.The total number of halogenated products likely to be formed by the ethane is:
Solution


Q6. The IUPAC name of (CH3 )3 C-CH=CH2 is:
Solution


Q7.Nine volumes of gaseous mixture consisting of gaseous organic compound A and just sufficient amount of oxygen required for complete combustion yielded on burning four volumes of CO_2,six volumes of water vapour, and two volumes of N_2, all volumes measured at the same temperature and pressure. If the compound contains C, H, and N only, the molecular formula of the compound A is:
Solution
58 (b) 2Cx Hy Nz+7O₂→ 4CO2+6H2 O+2N2
2x=4 x=2
2y =12 y=6
2z=4 z=2
Formula = C2 H6 N2
58 (b) 2Cx Hy Nz+7O₂→ 4CO2+6H2 O+2N2
2x=4 x=2
2y =12 y=6
2z=4 z=2
Formula = C2 H6 N2
Q8.Which of the following structures represents cyclopentyl methyl carbinol?
Solution


Q9.Hyperconjugation involves overlap of the following orbitals
Solution


Q10. The weight of 1 litre of ozonised oxygen at STP was found to be 1.5 gm. When 100 ml of this mixture at STP was treated with turpentine oil, the volume was reduced to90 ml.The molecular weight of ozone is:
Solution
(c) Volume of O3in 100 ml of ozonised O2
=100-90=10 ml (dissolved in turpentine)
Volume of O3 in 1 litre of ozonised O2= (10×100)/100=100 ml
Volume of O2in 1 litre =100 -100=900 ml
Weight of 900 ml of O2at STP =(900×32)/22400
= 1.286 gm
Weight of 100 ml O3 at STP =1.5-1.286
= 0.214 gm
Now, 100 ml of O_3at STP weighs = 0.214 gm
22400 ml of O3at STP weighs = (0.214×22400)/100
= 47.94 gm
Molecular weight of O3= 47.94 gm
(c) Volume of O3in 100 ml of ozonised O2
=100-90=10 ml (dissolved in turpentine)
Volume of O3 in 1 litre of ozonised O2= (10×100)/100=100 ml
Volume of O2in 1 litre =100 -100=900 ml
Weight of 900 ml of O2at STP =(900×32)/22400
= 1.286 gm
Weight of 100 ml O3 at STP =1.5-1.286
= 0.214 gm
Now, 100 ml of O_3at STP weighs = 0.214 gm
22400 ml of O3at STP weighs = (0.214×22400)/100
= 47.94 gm
Molecular weight of O3= 47.94 gm