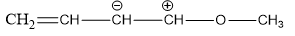IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
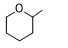
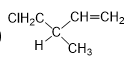
Q1. Which of the following is oxetane?called:
Solution
Part B
Part B
Q2.Which of the following compounds will not show geometrical isomerism?
Solution


Q3. The total number of alkenes possible by dehydromination of 3-bromo-3-cyclopentylhexane using alcoholic KOH is
Solution


Q4. An organic compound contains 66% C and 13.3% H. Its vapour density is 37. The possible number of isomers of all types for the compound is:
Solution


Q5.The total number of cyclic structural as well as stero isomers possible for a compound with the molecular formula C5 H10 is
Solution


Q6. Which of the following will not show geometrical isomerism?
Solution
(b)
In (b), the two groups (two H atoms) are same around the double bond
(b)
In (b), the two groups (two H atoms) are same around the double bond
Q7.In 3-chloro cyclohexanol, the primary prefix is:
Solution
Part B
Part B
Q8.Which of the following resonating structures of 1-methoxy-1, 3-butadiene is least stable?
Solution
(c) The octet of all atoms are complete in structures a and b. The molecule in which all the atoms have completed octet is more stable than atom which have incomplete octet. Larger the number of resonating structures, larger will be the stability, thus structures a and b are stable. In structure (d), the electron deficient of positive charged carbon is duly compensated by one pair electrons of adjacent oxygen atoms while such neighbour group support is not available in structure (c). Hence, structure (c) is least stable in comparison to structure (d).
(c) The octet of all atoms are complete in structures a and b. The molecule in which all the atoms have completed octet is more stable than atom which have incomplete octet. Larger the number of resonating structures, larger will be the stability, thus structures a and b are stable. In structure (d), the electron deficient of positive charged carbon is duly compensated by one pair electrons of adjacent oxygen atoms while such neighbour group support is not available in structure (c). Hence, structure (c) is least stable in comparison to structure (d).
Q9.Butene when treated with chlorine at about 500°C forms:
Solution
Part B
Part B
Q10. An organic compound on analysis gave C=42.8%,H=720%, and N=50%. Volume of 1 gm of the compound was found to be 200 ml at STP. Molecular formula of the compound is:
Solution