IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. 

Solution


Q2.

Solution


Q3. The number of isomers that can be obtained theoretically on monochlorination of 2-methylbutane is:
Solution
Part D
Part D
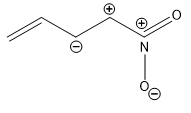
Q4. Among the following the least stable resonance structure is
Solution
(a) Two positive charges present at the adjacent place, elevates the energy, thus lowers the stability most.
(a) Two positive charges present at the adjacent place, elevates the energy, thus lowers the stability most.
Q5.Which of the following compounds exhibits stereoisomerism?
Solution


Q6. A mixture of ethylene and excess of H_2had a pressure of 600 mm Hg. The mixture was passed over nickel catalyst to convert ethylene to ethane. The pressure of the resultant mixture at the similar conditions of temperature and volume dropped to 400 mm Hg. The fraction of C_2 H_4 by volume in the original mixture is:
Solution


Q7.In Liebig's method for the estimation of C and H, if the compound also contains N, which of the following is kept near the exit of the combustion tube?
Solution
(d) On heating the compound N_2gas is evolved which is absorbed by Cu gauge
(d) On heating the compound N_2gas is evolved which is absorbed by Cu gauge
Q8.Which of the following carbocations is most stable?
Solution


Q9.The hybridisation of C atoms in (C-C) single-bond of H-C≡C-CH≡CH2 is:
Solution
Part C
Part C
Q10. Which of the following statements is correct?
Solution
(c) The presence of an asymmetric C atom is not essential, e.g., allenes of the type (RR’C=C=CRR’) are optically active although they do not contain chiral C atoms
(c) The presence of an asymmetric C atom is not essential, e.g., allenes of the type (RR’C=C=CRR’) are optically active although they do not contain chiral C atoms