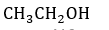IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
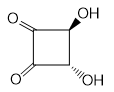
Q1. 1-Propanol and 2-propanol can be best distinguished by:
Solution


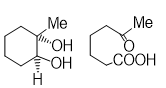
Q2.Which one of the following will most readily by dehydrated in acidic conditions?
Solution


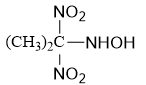
Q3. Compound (A)(→┴(2 mol of))┬(HIO_4 ) 2 mol of glyoxalic acid.The compound (A) is:
Solution


Q4. When KMnO_4 acts as an oxidizing agent and ultimately from MnO_4^(2-), MnO_2, Mn_2 O_3, and Mn^(2+) then the numbers of electrons transferred in each case, respectively, are
Solution
(c)
e^-+MnO_4^⊖⟶MnO_4^( 2-)
3e^-+MnO_4^⊖⟶MnO_2
4e^-+2MnO_4^⊖⟶Mn_2 O_3
5e^-+MnO_4^⊖⟶Mn^(2+)
(c)
e^-+MnO_4^⊖⟶MnO_4^( 2-)
3e^-+MnO_4^⊖⟶MnO_2
4e^-+2MnO_4^⊖⟶Mn_2 O_3
5e^-+MnO_4^⊖⟶Mn^(2+)
Q5.The number of moles of K_2 Cr_2 O_7 reduced by 1 mol of Sn^(2+) is
Solution
(c) Eq of Cr_2 O_7^(2-)≡Eq ofSn^(2+) (n=6)(n=2) 1/6 mol=1/2 mol 1/3 mol of Cr_2 O_7^(2-)=1 mol ofSn^(2+)
(c) Eq of Cr_2 O_7^(2-)≡Eq ofSn^(2+) (n=6)(n=2) 1/6 mol=1/2 mol 1/3 mol of Cr_2 O_7^(2-)=1 mol ofSn^(2+)
Q6. Which of the following acids possesses oxidising, reducing, and complex forming properties?
Solution
(d) In HNO_2, the oxidation state of N is +3. So it can go to a higher or lower oxidation state
(d) In HNO_2, the oxidation state of N is +3. So it can go to a higher or lower oxidation state
Q7.

Solution


Q8.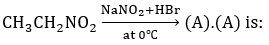
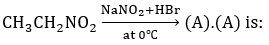
Solution


Q9.The oxidation states of sulphur in the anions SO_3^(2-),S_2 O_4^(2-)andS_2 O_6^(2-) follow the order
Solution
(a)
S_2 O_6^(2-):2x-12=-2⇒x=5
SO_3^(2-):x-6=-2⇒x=4
S_2 O_4^(2-):2x-8=-2⇒x=3
(a)
S_2 O_6^(2-):2x-12=-2⇒x=5
SO_3^(2-):x-6=-2⇒x=4
S_2 O_4^(2-):2x-8=-2⇒x=3
Q10. In Which of the following cases is the oxidation state of N atom wrongly calculated?
Compound Oxidation state
Compound Oxidation state
Solution