IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. The difference in the oxidation numbers of the two types of sulphur atoms in Na_2 S_4 O_6 is
Solution


Q2.The oxidation state of S in H_2 S_2 O_8 is
Solution
(c) H_2 S_2 O_8: It is peroxodisulphuric acid. So oxidation state of S=+6
(c) H_2 S_2 O_8: It is peroxodisulphuric acid. So oxidation state of S=+6
Q3. The oxidation state of Fe in Fe_3 O_8 is
Solution
(d) Fe_3 O_8 3x-16=0⇒x=16/3
(d) Fe_3 O_8 3x-16=0⇒x=16/3
Q4. In the balanced chemical reaction
IO_3^⊖+aI^⊖+bH^⊖⟶cH_2 O+dI_2
a,b,c, and d, respectively, correspond to
IO_3^⊖+aI^⊖+bH^⊖⟶cH_2 O+dI_2
a,b,c, and d, respectively, correspond to
Solution


Q5.Fehling’s solution can make distinction between:
Solution
(a) a.Fehling''''''''s solution reacts only with aliphatic aldehyde, not with aromatic aldehyde b.F.S. reacts with MeCHO and also with α-hydroxy ketones c.F.S. reacts with both HCHO and with α-hydroxy ketones d.F.S. reacts with both aliphatic aldehydes (MeCHOand HCHO). So the answer is (a)
(a) a.Fehling''''''''s solution reacts only with aliphatic aldehyde, not with aromatic aldehyde b.F.S. reacts with MeCHO and also with α-hydroxy ketones c.F.S. reacts with both HCHO and with α-hydroxy ketones d.F.S. reacts with both aliphatic aldehydes (MeCHOand HCHO). So the answer is (a)
Q6.
Solution
(d)
Disproportionation involves simultaneous oxidation and reduction of the same atom in a molecule
(d)
Disproportionation involves simultaneous oxidation and reduction of the same atom in a molecule
Q7.

Solution
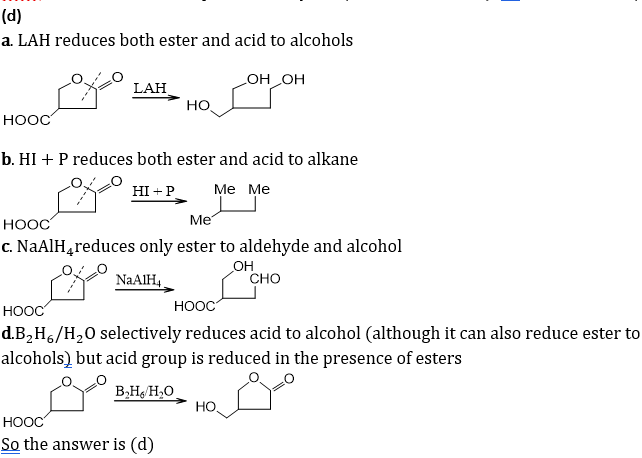

Q8.Which acetaldehyde is heated with Fehling’s solution, it gives a precipitate of:
Solution
Part C
Part C
Q9.In which of the following is the highest oxidation state not possible?
Solution
(b) Xe shows +8 oxidation state inXeF_6, but it does not exist because of steric hindrance of 8 F atoms
(b) Xe shows +8 oxidation state inXeF_6, but it does not exist because of steric hindrance of 8 F atoms
Q10. 

Solution
(a)
Equivalent of MnO_4^⊖= Equivalent ofFe (C_2 O_4 )
(n=5) (n=3)
(a)
Equivalent of MnO_4^⊖= Equivalent ofFe (C_2 O_4 )
(n=5) (n=3)













