IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. 

Solution
(d) In CN^⊖, oxidation of C is +2, and it changes to +4 oxidation state in CO_2. So C is also oxidised
(d) In CN^⊖, oxidation of C is +2, and it changes to +4 oxidation state in CO_2. So C is also oxidised
Q2.The oxidation number of P in Mg_2 P_2 O_7 is
Solution
(c) Mg_2 P_2 O_7:2×2+2x-14=0⇒x=5 Oxidation number of P=+5
(c) Mg_2 P_2 O_7:2×2+2x-14=0⇒x=5 Oxidation number of P=+5
Q3. 

Solution
(c) In (a), aqueous KMnO_4 (cold) will hydroxylate the (C=C) bond
In (b), NaOI (iodoform reaction) will not react since there is no (MeCO-) or (MeCHO)or (MeCH OH) group
In (c), Tollens reagent will convert the (-CHO) group to ( COO^⊖) group without affecting (C=C) bond Ph-CH=CH-CHO (→┴((i)Tollens reagent))┬((ii) H_3 O^⊕ ) Ph-CH=CH-COOH
In (d), MnO_2 will not react, since it does not contain allylic or benzylic alcoholic group. So the answer is (c)
(c) In (a), aqueous KMnO_4 (cold) will hydroxylate the (C=C) bond
In (b), NaOI (iodoform reaction) will not react since there is no (MeCO-) or (MeCHO)or (MeCH OH) group
In (c), Tollens reagent will convert the (-CHO) group to ( COO^⊖) group without affecting (C=C) bond Ph-CH=CH-CHO (→┴((i)Tollens reagent))┬((ii) H_3 O^⊕ ) Ph-CH=CH-COOH
In (d), MnO_2 will not react, since it does not contain allylic or benzylic alcoholic group. So the answer is (c)
Q4. Which of the following compounds is oxidized to prepare methyl ethyl ketone?
Solution


Q5.The oxidation number of carbon in CH_2 Cl_2 is
Solution
(a) CH_2 Cl_2:x+2-2=0⇒x=0 Oxidation state of C=0
(a) CH_2 Cl_2:x+2-2=0⇒x=0 Oxidation state of C=0
Q6. 

Solution
(b) MnO_2 selectively oxidises allylic or benzylic hydroxyl group to (C=O) group, whereas Jones reagent oxidises 1° and 2°ROH to aldehydes and ketones, respectively. So, the answer is (b)
(b) MnO_2 selectively oxidises allylic or benzylic hydroxyl group to (C=O) group, whereas Jones reagent oxidises 1° and 2°ROH to aldehydes and ketones, respectively. So, the answer is (b)
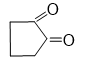
Q7.The oxidation product of 1,2-cyclopentane diol with HIO_(4 )or (CH_3 〖COO)〗_4 Pb is:
Solution


Q8.The oxidation state of chromium in the final product formed in the reaction between KI and acidified potassium dichromate solution is
Solution
(d)
6e^-+Cr_2 O_7^(2-)⟶2Cr^(3+)
2I^⊖⟶I_2+2e^-
Oxidation state of Cr=+3
(d)
6e^-+Cr_2 O_7^(2-)⟶2Cr^(3+)
2I^⊖⟶I_2+2e^-
Oxidation state of Cr=+3
Q9.

Solution
(d) II,III and IV are redox reactions
(d) II,III and IV are redox reactions
Q10. The number of moles of KMnO_4 that will be needed to react with 1 mol of sulphite ion in acidic solution is
Solution