IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. Of the following compounds, whose ozonolysis proves the Kekule structure of benzene?
Solution
(c) o-Xylene on ozonolysis gives glyoxal: methylglyoxal: dimethyl glyoxal in the ratio 3:2:1, which proves the existence of resonance in benzene and hence proves the Kekule structure of benzene. So the answer is (c)
(c) o-Xylene on ozonolysis gives glyoxal: methylglyoxal: dimethyl glyoxal in the ratio 3:2:1, which proves the existence of resonance in benzene and hence proves the Kekule structure of benzene. So the answer is (c)
Q2.

Solution
(a) NH_4HS selectively reduces (-NO_2) group parato the (Me) (EDG) group, whereas (SnCl_2+HCl) selectively reduces (-NO_2) group orthoto the (Me) (EDG) group. So the answer is (a)
(a) NH_4HS selectively reduces (-NO_2) group parato the (Me) (EDG) group, whereas (SnCl_2+HCl) selectively reduces (-NO_2) group orthoto the (Me) (EDG) group. So the answer is (a)
Q3. An oxidation process involves
Solution
(a) The statement is self-explanatory
(a) The statement is self-explanatory
Q4. 

Solution


Q5.The equivalent weight of MnSO_4 is Half its molecular weight when it is converted to
Solution
(b)
∴ Equivalent weight =M/2
(b)
∴ Equivalent weight =M/2
Q6. Caprolactone on reduction with LAH or H_2 + Pt or Pd gives:
Solution


Q7.The oxidation number of S in Na_2 S_4 O_6 is
Solution
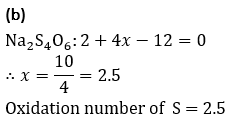
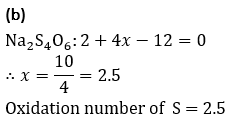
Q8.

Solution
Part B
Part B
Q9.

Solution


Q10. 

Solution
(a) In (A) the oxidation of allylic1°alcohol to aldehyde is carried out with MnO_2, while in (B) the oxidation of 2° alcohol to ketone is carried out with CrO_3+CH_3 COOH. So the answer is (a)
(a) In (A) the oxidation of allylic1°alcohol to aldehyde is carried out with MnO_2, while in (B) the oxidation of 2° alcohol to ketone is carried out with CrO_3+CH_3 COOH. So the answer is (a)








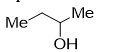




 >
>


