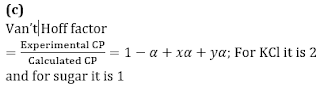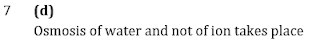IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. The ratio of the value of any colligative property for KCl solution to that of sugar solution is:
Q2.What would be the freezing point of aqueous solution containing 17 g of C2 H5 OH in 1000 g of water. K(fH2 O)=1.86 K m(-1)
Q3. The osmotic pressure of a solution (density is 1 g mL(-1)) containing 3 g of glucose (molecular weight = 180) in 60 g of water at 15℃ is
Q4. The molal freezing point constant of water is 1.86 K m(-1), If 342 g of cane sugar (C12 H22 O11) is dissolved in 1000 g of water, the solution will freeze at
Q5.On mixing 10 mL of acetone with 40 mL of chloroform, the total volume of the solution is
Q6. The most likely of the following mixtures to be an ideal solution is
Solution
a) NaCl-H2 O
a) NaCl-H2 O
Q7.FeCl3 on reaction with K4 [Fe(CN)6 ] in aqueous solution gives blue colour. These are separated by a semi-permeable membrane AB as shown. Due to osmosis, there is
Q8.A mixture of volatile components A and B has total vapour pressure (in torr)
P=254 -119χA
Where χA is the mole fraction of A in mixture. Hence, PA° and PB° are (in torr)
Q9.An azeotropic solution of two liquids has boiling point lower than either of them when it