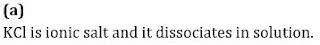IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
This section contain(s) 10 questions numbered 1 to 10. Each question contains STATEMENT 1(Assertion) and STATEMENT 2(Reason). Each question has the 4 choices (a), (b), (c) and (d) out of which ONLY ONE is correct..
Q1. Statement 1: One molar aqueous solution has always higher concentration than one molal
Statement 2: The molality of a solution depends upon the density of the solution whereas molarity does not
Q1. Statement 1: One molar aqueous solution has always higher concentration than one molal
Statement 2: The molality of a solution depends upon the density of the solution whereas molarity does not
Q2.Statement 1: An ideal solution is one which obey Raoult’s law.
Statement 2: KCl(aq) is an ideal solution.
Statement 2: KCl(aq) is an ideal solution.
Q3. Statement 1: The molecular weight of acetic acid determined by depression in freezing point method in benzene and water was found to be different.
Statement 2: Water is polar and benzene is non polar.
Statement 2: Water is polar and benzene is non polar.
Q4. Statement 1: At low concentration, benzene and toluene forms ideal solution.
Statement 2: Components with structural similarity forms ideal solution.
Q5.Statement 1: Osmosis is one sided movement of solvent particles.
Statement 2: In osmosis, the net movement of solvent particles from dil. to conc. solution and from conc.to dil. solution takes place through semipermeable membrane, showing finally the direction of dil. to conc.
Statement 2: In osmosis, the net movement of solvent particles from dil. to conc. solution and from conc.to dil. solution takes place through semipermeable membrane, showing finally the direction of dil. to conc.
Q6. Statement 1: ∆Hmix and ∆Vmix in an ideal solution are zero
Statement 2: A-B interactions in ideal solutions are same as between A-B and B-B
Statement 2: A-B interactions in ideal solutions are same as between A-B and B-B
Q7.Statement 1: The molecular mass of polymers cannot be calculated using the boiling point or freezing point method
Statement 2: The boiling point method for determining the molecular masses is used for compounds stable at high temperature
Statement 2: The boiling point method for determining the molecular masses is used for compounds stable at high temperature