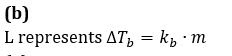IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
This section contain(s) 10 paragraph(s) and based upon each paragraph, multiple choice questions have to be answered. Each question has atleast 4 choices (a), (b), (c) and (d) out of which ONLY ONE is correct..
Q1. The vapour pressure of the solution is comparatively smaller than that of the pure solvent (Raoult’s law). Consequently, the temperature at which the vapour pressure of solution becomes equal to the external pressure, will be greater than that of pure solvent, rising its boiling point. Since, the decrease in vapour pressure is directly proportional to the amount fraction of the solute in the solution, it is therefore expected that the corresponding increase in the boiling points also depends on the amount fraction of the solute in the solution
When a non-volatile solute is dissolved in a solvent, the relative lowering of vapour pressure is equal to
Q1. The vapour pressure of the solution is comparatively smaller than that of the pure solvent (Raoult’s law). Consequently, the temperature at which the vapour pressure of solution becomes equal to the external pressure, will be greater than that of pure solvent, rising its boiling point. Since, the decrease in vapour pressure is directly proportional to the amount fraction of the solute in the solution, it is therefore expected that the corresponding increase in the boiling points also depends on the amount fraction of the solute in the solution
When a non-volatile solute is dissolved in a solvent, the relative lowering of vapour pressure is equal to
Q2.The variation of vapour pressure of the solvent and that of the solution with temperature are given by the respective solvent-vapour and solution-vapour curves of the phase diagramFor a given value of the external pressure, the pure solvent will boil at temperature Tb* and at temperature Tb let pext be equal to p*, the vapour pressure of pure solvent
Applying the Clausius-Clapeyron equation to the solution vapour equilibrium for the two values of p,Tb* and p*,Tb we have
In p*/p=(∆vap H1m)/R (1/(Tb* )-1/Tb )
=(∆vap H1m)/R (∆Tb)/(Tb* Tb )
The phase diagram for the pure solvent and solution are recorded below. The quantity indicated by L in the figure is
The phase diagram for the pure solvent and solution are recorded below. The quantity indicated by L in the figure is
Q3. An aqueous solution freezes at 272.4 K while pure water freezes at 273 K. Given, Kf=1.86 K kg mol(-1),Kb=0.512 K kg mol(-1) and vapour pressure of water at 298 K = 23.756 mm of Hg. Determine the following
Molality of the solution is
Molality of the solution is
Q4. A solution of sucrose (molar mass =342) is prepared by dissolving 68.4 g in 1000 g of water. Calculate
The vapour pressure of solution at 293 K
Q5.The osmotic pressure π depends on the molar concentration of the solution (π=CRT). If two solutions are of equal solute concentration and, hence, have the same osmotic pressure, they are said to be isotonic. If two solutions are of unequal osmotic pressures, the more concentrated solution is said to be hypertonic and the more diluted solution is described as hypertonic.
Osmosis is the major mechanism for transporting water upward in the plants. Answer the following questions:
A plant cell shrinks when it is kept in:
A plant cell shrinks when it is kept in:
Q6. The electrolyte solutions show abnormal colligative properties. To account for this effect we define a quantity called the Van’t Hoff factor given by
i=(Actual number of particles in solution after dissociation)/(Number of formula units initially dissolved in solution)
i=1 (for non-electrolytes)
i> 1 (for electrolytes, undergoing dissociation)
i< 1 (for solutes, undergoing association)
Answer the following questions:
Benzoic acid undergoes dimerization in benzene solution. The Van’t Hoff factor i is related to the degree of association α of the acid as
Benzoic acid undergoes dimerization in benzene solution. The Van’t Hoff factor i is related to the degree of association α of the acid as
Q7.
Compartments A and B have the following combinations of solution:
Indicate the number of solutions which is/are isotonic
Compartments A and B have the following combinations of solution:
Indicate the number of solutions which is/are isotonic
Q8.The boiling point elevation and freezing point depression of solutions have a number of practical applications. Ethylene glycol (CH2 OH-CH2 OH) is used in automobile radiators as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3 OH), a fairly volatile liquid that boils only at 65℃, is sometimes used as an antifreeze in automobile radiators
Which of the following is a better reagent for depression in freezing point but not for elevation in boiling point?
Which of the following is a better reagent for depression in freezing point but not for elevation in boiling point?
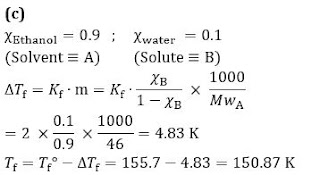
Q9.A solution M is prepared by mixing ethanol and water, the mole fraction of ethanol in the mixture is 0.9
Given : Freezing point depression constant of water
(Kfwater )=1.86 K kg mol(-1)
Freezing point depression constant of ethanol
(Kfethanol )=2.0 K kg mol(-1)
Boiling point elevation constant of water
(Kbwater )=0.52 K kg mol(-1)
Boiling point elevation constant of ethanol
(Kbethanol )=1.2 K kg mol(-1)
Standard freezing point of water = 273 K
Standard freezing point of ethanol = 155.7 K
Standard boiling point of water = 373 K
Standard boiling point of ethanol = 351.5 K
Vapour pressure of pure water = 32.8 mm Hg
Vapour pressure of pure ethanol = 40 mm Hg
Molecular weight of water = 18 g mol(-1)
Molecular weight of ethanol = 46 g mol(-1)
In answering the following questions consider the solutions to be ideal dilute solutions and solutes to be non-volatile and non-dissociative
The freezing point of the solution M is
The freezing point of the solution M is
Q10.Properties such as boiling point, freezing point, and vapour pressure of a pure solvent change when solute molecules are added to get homogenous solution. These are called colligative properties
Answer the following questions:
I 0.001 m NaCl II 0.001 m urea III 0.001 m MgCl_2 IV 0.001 m CH_3 COOH Increasing order of boiling points
I 0.001 m NaCl II 0.001 m urea III 0.001 m MgCl_2 IV 0.001 m CH_3 COOH Increasing order of boiling points