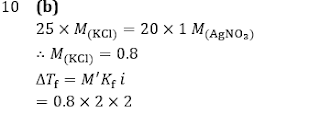IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q2.Two solutions of KNO3 and CH3COOH are prepared separately. The molarity of both is 0.1 M and osmotic pressure P1 and P2 respectively. The correct relationship between the osmotic pressures is
Q4. Each pair forms ideal solution except
Q5.The Henry’s law constant for the solubility of N2 gas in water at 298 K is 1.0 × 105 atm. The mole fraction of N2 In air is 0.8 The number of moles of N2 from air dissolved in 10 moles of water of 298 K and 5 atm pressure is
Q6. The use of common salts, e.g NaCl or CaCl2 anhydrous is made to clear snow on the rods. This causes:
Q7.The relative decrease in the vapour pressure of an aqueous solution containing 2 mol [Cu(NH3 )3 Cl]Cl in 3 mol H2 O is 0.50. On reaction with AgNO3, this solution will form
Q8.Which of the following 0.1 M aqueous solutions will have the lowest freezing point?
Q10. 25 mL of an aqueous solution of KCl was found to require 20 mL of 1 M AgNO3 solution when titrated using a K2 CrO4 as indicator. The depression in freezing point of KCl solution with 100% ionization will be:
(Kf=2.0° mol(-1) kg and molarity = molality)