IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1.1.6 g of pyrousite ore was ore was treated with 50 mL of 0.1 N oxalic acid and some sulphuric acid. the oxalic acid left in excess was raised to 250 mL in a flask. 25 mL of this solution, when titrated with 0.01 NKMnO_4, required 30 mL of the solution. The percentage of pure MnO_2 in the sample is
Solution


Q2.How many moles of electrons weight one kilogram?
Solution


Q3. Which of the following has maximum number of C-atoms?
Solution


Q4. Dimethyl glyoxime and NaHSO_3/NH_4 CNS are used to distinguish and separate Cu^(2+) and Ni^(2+). These are used to order
Solution


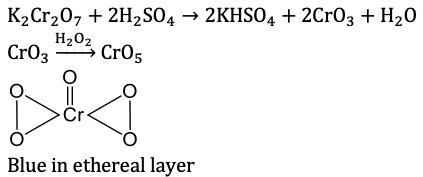
Q5. K_2 Cr_2 O_7+conc.H_2 SO_4+H_2 O_2+ ether → blue perchromic anhydride (in ethereal layer). Blue colour is due to
Solution

Q6. 1.2 g of Mg is treated with 100 mL of 1 M H_2 SO_4.Molar concentration of the H_2 SO_4 solution after complete reaction is
Solution


Q7. Number of moles in 1.8 g H_2 O is equal to the number of moles in I : 1.8 g glucose II : 6 g urea III : 34.2 g sucrose Select the correct group.
Solution


Q8. K_2 CrO_4oxidises KI in the presence of HCl to I_2. The equivalent weight of the K_2 CrO_4 is
Solution


Q9. Aqueous solution of salt

Solution


Q10. A sample of CaCO_3 is 50% pure. On heating 1.12 L of CO_2 (at STP) is obtained. Residue left (assuming non-volatile impurity) is
Solution







