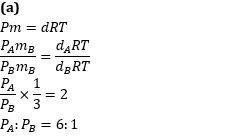IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything.
Q1. The density of a gas A is twice that of a gas B at the same temperature. The molecular mass of gas B is thrice that of A. The ratio of the pressure acting on A and B will be
Q2. The ratio of the rate of diffusion of helium and methane under identical condition of pressure and temperature will be
Q3. Statement 1: At low pressure, van der Waals’ equation is reduced to [P+a/V2] V = RT.
Statement 2: The compressibility factor corresponding to low pressure is given by 1-RTV/a
Statement 2: The compressibility factor corresponding to low pressure is given by 1-RTV/a
Q4. Statement 1: Most probable velocity is the velocity possessed by maximum fraction of molecules at the same temperature
Statement 2: On collision, more and more molecules acquire higher speed at the same temperature
Statement 2: On collision, more and more molecules acquire higher speed at the same temperature
Q5. Statement 1: SO2 gas is easily liquefied while H2 is not
Statement 2: SO2 has low critical temperature while H2 has high critical temperature
Statement 2: SO2 has low critical temperature while H2 has high critical temperature
Q6. Statement 1: On cooling, the brown colour of nitrogen dioxide disappears
Statement 2: On cooling, NO2 undergoes dimerization resulting in the pairing of the odd electron in NO2
Statement 2: On cooling, NO2 undergoes dimerization resulting in the pairing of the odd electron in NO2
Q7.Statement 1: The pressure of a fixed amount of an ideal gas is proportional to its temperature
Statement 2: Frequency of collisions and their impact both increase in proportion of the square root of temperature
Statement 2: Frequency of collisions and their impact both increase in proportion of the square root of temperature
Q8.Statement 1: The value of van der Waals constant a is larger for ammonia than for nitrogen
Statement 2: Hydrogen bonding is present in ammonia
Statement 2: Hydrogen bonding is present in ammonia
Q9.Statement 1: The hot air balloons in sports and for meteological observations is an application Charles law.
Statement 2: Hot air is less dense and hence gases expand on heating.
Statement 2: Hot air is less dense and hence gases expand on heating.
Q10. Statement 1: Helium shows only positive deviations from ideal behaviour
Statement 2: Helium is an inert gas
Statement 2: Helium is an inert gas