IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything.
Q1. Two flasks A and B have equal volume. A is maintained at 300 K and B at 600 K. WhileA contains H2 gas, B has an equal mass of CH4 gas. Assuming ideal behaviours for both the
gases, answer the following:
Flask containing greater number of molecules
Flask containing greater number of molecules
Q2. The van der Waals constant for gases A,B, and C are as follows:
Gas a(dm6 kPa mol-2) b(dm3 mol-1) A 405.3 0.027 B 1215.9 0.030 C 607.95 0.032 Answer the following:
Which gas has the highest critical temperature?
Gas a(dm6 kPa mol-2) b(dm3 mol-1) A 405.3 0.027 B 1215.9 0.030 C 607.95 0.032 Answer the following:
Which gas has the highest critical temperature?
Q3. For the given ideal gas equation PV=nRT, answer the following questions:
In the above equation, the value of universal gas constant depends only upon
In the above equation, the value of universal gas constant depends only upon
Q4. Using van der Waals equation (P+a/V2)(V-b)=RT, answer the following equations:
The van der Waals equation explains the behaviour of
The van der Waals equation explains the behaviour of
Q5. Compressibility factor Z=PV/RT. Considering ideal gas, real gas, and gases at critical state, answer the following questions:
The compressibility factor of an ideal gas is
The compressibility factor of an ideal gas is
Q6. Two gaseous molecules A and B are traveling towards each other. Let the mean free path of the molecule be σ and Z be the collision number with other molecules at pressure 1 atm.
Answer the following questions
The free path of a gas molecule is the distance
The free path of a gas molecule is the distance
Q7. The constant motion and high velocities of gas particles lead to some important practical consequences. One such consequence is that gases mix rapidly when they come in contact.
Take the stopper off a bottle of perfume, for instance, and the odour will spread rapidly through the room as perfume molecules mix with the molecules in the air. This mixing of
different gases by random molecular motion and with frequent collision is called diffusion. A similar process in which gas molecules escape without collision through a tiny hole into a
vaccum is called effusion. Both the processes follow Graham’s law which is mathematically put as r ∝ √1/d. The average distance travelled by molecules between successive collisions
is called mean free path.
Answer the following questions on the basis of the above information:
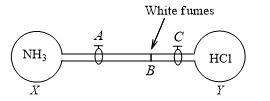
The stopcocks of the bulbs X (containing NH3) and Y (containing HCl), both under identical conditions, are opened simultaneously. White fumes of NH4Cl, are formed at point B. If AB =36.5 cm, then BC is approximately
Answer the following questions on the basis of the above information:
The stopcocks of the bulbs X (containing NH3) and Y (containing HCl), both under identical conditions, are opened simultaneously. White fumes of NH4Cl, are formed at point B. If AB =36.5 cm, then BC is approximately
Q8. The behaviour of ideal gas is governed by various gas laws which are described by mathematical statements as given below:
1. PV=k (constant) at constant n and T
2. V⁄T = k2 (constant) at constant n and P
3. V⁄n = k3 (constant) at constant T and P
4. PV = nRT
5. P⁄T = k4(constant) at constant n and V Answer the following
The value of k2 is
1. PV=k (constant) at constant n and T
2. V⁄T = k2 (constant) at constant n and P
3. V⁄n = k3 (constant) at constant T and P
4. PV = nRT
5. P⁄T = k4(constant) at constant n and V Answer the following
The value of k2 is
Q9.Consider the adjacent diagram. Initially, flask A contained oxygen gas at 27℃ and 950 mm of Hg, and flask B contained neon gas at 27℃ and 900 mm. Finally, two flask were joined by
means of a narrow tube of negligible volume equipped with a stopcock and gases were allowed to mixup freely. The final pressure in the combined system was found to be 910 mm of
Hg
Which of the following statements concerning oxygen and neon gas is true in the beginning, when the stopcock was just opened?
Which of the following statements concerning oxygen and neon gas is true in the beginning, when the stopcock was just opened?
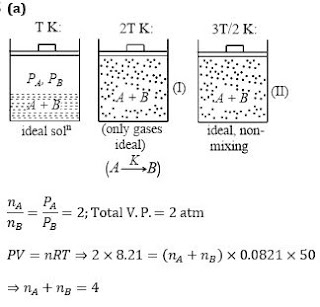
Q10. The system shown in the figure is in equilibrium, where A and B are isomeric liquids and form an ideal solution at T K. Standard vapour pressures of A and B are P0A and P0B ,
respectively, at T K. We collect the vapour of A and B in two containers of volume V, first container is maintained at 2 T K and second container is maintained at 3T⁄2. At the
temperature greater than T K, both A and B exist in only gaseous form,
We assume than collected gases behave ideally at 2 T K and there may take place an isomerization reaction in which A gets converted into Bby first-order kinetics reaction given as:
where k is a rate constant.
In container (II) at the given temperature 3T/2, A and B are ideal in nature and non reacting in nature. A small pin hole is made into container. We can determine the initial rate of effusion of both gases in vacuum by the expression
r=K∙P/√M0 Where, P=pressure difference between system and surrounding K=positive constant M0=molecular weight of the gas
If partial vapour pressure of A is twice that of partial vapour pressure of B and total pressure 2 atm at T K, where T=50 K and V=8.21 L, then the number of moles of A and B in vapour phase is:
We assume than collected gases behave ideally at 2 T K and there may take place an isomerization reaction in which A gets converted into Bby first-order kinetics reaction given as:
where k is a rate constant.
In container (II) at the given temperature 3T/2, A and B are ideal in nature and non reacting in nature. A small pin hole is made into container. We can determine the initial rate of effusion of both gases in vacuum by the expression
r=K∙P/√M0 Where, P=pressure difference between system and surrounding K=positive constant M0=molecular weight of the gas
If partial vapour pressure of A is twice that of partial vapour pressure of B and total pressure 2 atm at T K, where T=50 K and V=8.21 L, then the number of moles of A and B in vapour phase is: