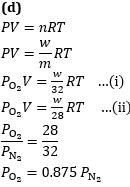IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything.
Q2. A bottle of dry ammonia and a bottle of dry hydrogen chloride connected through a long tube are opened simultaneously at both ends. The white ammonium chloride ring first formed will be
Q3. Equal weights of ethane and hydrogen are mixed in an empty container at 25℃. The fraction of the total pressure exerted by hydrogen is
Q4. A vessel is filled with a mixture of oxygen and nitrogen. At what ratio of partial pressures will the mass of gases be identical?
Q7.2 mol H2 is mixed with 2 gm of H2. The molar heart capacity at constant pressure for the mixture is
Q8.If v is the volume of one molecule of a gas under given conditions, then van der Waals constant b is
Q9.When an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
Q10. A gas obeys P(V-b)=RT. Which of the following are correct about this gas?
1. Isochoric curves have slope = R/(V-b)
2. Isobaric curves have slope R/P and intercept b
2. For the gas compressibility factor = 1+Rb/RT
2. The attraction forces are overcome by repulsive forces
- Isochoric curves have slope = R/(V-b)
- Isobaric curves have slope R/P and intercept b
- For the gas compressibility factor = 1+Rb/RT
- The attraction forces are overcome by repulsive forces