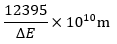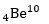IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. Beta-emission takes place
Solution
Part D
Part D
Q2.The angular momentum of an electron in 4s orbital, 3p orbital, and 4th orbit are
Solution
(a)
2K, 8L, 9M, and 2N 1s^2 2s^2 2p^6 3s^2 3p^6 3d^1 4s^2 (■(K means n=1,L means n=2 @M means n=3,N means n=4 )) Structure is 3d^1,4s^2
Atomic number 21
The total number of pe^-
2p^6+3p^6=12
(a)
2K, 8L, 9M, and 2N 1s^2 2s^2 2p^6 3s^2 3p^6 3d^1 4s^2 (■(K means n=1,L means n=2 @M means n=3,N means n=4 )) Structure is 3d^1,4s^2
Atomic number 21
The total number of pe^-
2p^6+3p^6=12
Q3. Slow neutrons can bring about the fission of
Solution
Part A
Part A
Q4. When passing through a magnetic field the largest deflection is experienced by
Solution
Part B
Part B
Q5.

Solution


Q6. 4 Be^7captures a K electron into its nucleus. What is the mass number and atomic number of the nuclide formed?
Solution
(a)
4Be^7+ (-1) e^0 → 3 Li^7 So, atomic number=3, mass number=7
(a)
4Be^7+ (-1) e^0 → 3 Li^7 So, atomic number=3, mass number=7
Q7.When electronic transition occurs from higher energy state to lower energy state with energy difference equal to ΔE electron volts, the wavelength of the line emitted is approximately equal to
Solution


Q8.Which of the following nuclei is unstable?>
Solution
Part
Part
Q9.Thiosulphate ion, S2O3^(2-) on acidification changes to SO_2 along with precipitation of sulphur
^35 S^32 SO_3^(2-)+2H^+→H_2 O+SO_2+S
Which is the correct statement?
^35 S^32 SO_3^(2-)+2H^+→H_2 O+SO_2+S
Which is the correct statement?
Solution
Part
Part
Q10. The electrons, identified by quantum numbers n and l
n=4,l=1
n=4,l=0
n=3,l=2
n=3,l=1
Can be placed in the order of increasing energy, from the lowest to highest, as
n=4,l=1
n=4,l=0
n=3,l=2
n=3,l=1
Can be placed in the order of increasing energy, from the lowest to highest, as
Solution