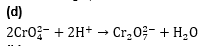IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1. Which represents correct comparison of the stability of ions?
Solution
d
d
Q2.Ni2+ in traces, can be tested using
Solution
b
b
Q3. Consider a titration of potassium dichromate solution with acidified Mohr’s salt solution using diphenylamine as indicator. The number of moles of Mohr’s salt required per mole of dichromate is
Solution
d
d
Q4. At pH=4,Cr2 O42- exists as
Q5.Which is not the true statement?
Solution
c
c
Q6. The oxides, CrO3,MoO3 and WO3 are strongly
Q7.Which of the following transition element shows the highest oxidation state?
Solution
a
a
Q8.On addition of AgNO3 to four different test tubes containing different solutions, one of them gave a white precipitate. It may be
Solution
b
b
Q9.K3[Fe(CN)6] is used as an external indicator in the dichromate estimation of Fe2+. Following change is observed
Solution
d
d
Q10. A solution of a metal ion when treated with KI gives a red precipitate which dissolves in excess KI to give a colourless solution. Moreover, the solution of metal ion on treatment with a solution of cobalt (II) thiocyanate gives rise to a deep blue crystalline precipitate. The metal ion is
Solution
b
b