IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1.Nitration of aromatic compounds is done using a mixture of conc. H2SO4 and conc. HNO3. Intermediate formed in the nitration process is
Q2.In which case hydrolysis is faster
Solution
Since Si-H and Si-D bonds are not affected, hence hydrolysis takes place at equal rates
Since Si-H and Si-D bonds are not affected, hence hydrolysis takes place at equal rates
Q4.Hydrazine is not
Solution
An acid
In hydrazine, N is not in highest oxidation state so it can work as oxidising agent as well as reducing agent. Also N has lone pair of electrons so its a lewis base.
An acid
In hydrazine, N is not in highest oxidation state so it can work as oxidising agent as well as reducing agent. Also N has lone pair of electrons so its a lewis base.
Q5.NaOH can’t be stored in
Solution
NaOH reacts with Al and Zn both thus cannot be stored in the vessel made of Al or Zn
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Zn + 2NaOH → Na2ZnO2 + H2
NaOH reacts with Al and Zn both thus cannot be stored in the vessel made of Al or Zn
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Zn + 2NaOH → Na2ZnO2 + H2
Q6.Which of the following compounds do not exist?
N4, P4, PCl5, NCl5, NCl3, P2O5, NO2, PO2
Solution
N4, NCl5, PO2
NCl5 does not exist bcoz nitrogen atom does not have d-orbitals to accomodate electrons from chlorine atoms and nitrogen cant accomodate more than 8 electrons in its valence shell. so its covalency exists only upto four
N − 2s2p3
P − 3s2p3 but as P can expand its valency using vacant d-orbitals so maximum valency of N & P are 3 and 5.
So N4 doesnt exist.
Also, P doesnt form divalent compounds so PO2 doesnt exist.
N4, NCl5, PO2
NCl5 does not exist bcoz nitrogen atom does not have d-orbitals to accomodate electrons from chlorine atoms and nitrogen cant accomodate more than 8 electrons in its valence shell. so its covalency exists only upto four
N − 2s2p3
P − 3s2p3 but as P can expand its valency using vacant d-orbitals so maximum valency of N & P are 3 and 5.
So N4 doesnt exist.
Also, P doesnt form divalent compounds so PO2 doesnt exist.
Q7.Which forms pπ-pπ multiple bonds with itself and with C and O?
Solution
C≡N, N≡N, O ← N=O
C≡N, N≡N, O ← N=O
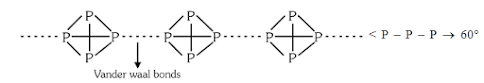
Q8.The high reactivity and low volatility of white phosphorus is due to
Q9.Lead as impurity in the extraction of silver is removed by
Solution
Parke’s process
In this process, molten zinc is added to mineral when silver is extracted into zinc in larger quantity than lead
Parke’s process
In this process, molten zinc is added to mineral when silver is extracted into zinc in larger quantity than lead
Q10. Boron sesquioxide is
Solution
B2O3
B2O3