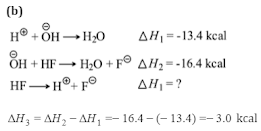IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1.1 mol of NH3gas at 27℃ is expanded under adiabatic condition to make volume 8 times (γ=1.33). Final temperature and work done, respectively, are:
Q2.Which of the following equations corresponds to the definition of enthalpy of formation at 298 K?
Solution
In enthalpy of formation, reactants and products must be in most stable standard state
In enthalpy of formation, reactants and products must be in most stable standard state
Q3.Heat of neutralization of CsOH with all strong acid is 13.4 kcal mol-1. The heat released on neutralization of CsOH with HF (weak acid)is 16.4 kcal mol-1. ∆H⊝of ionization of HF is
Q4.Inversion temperature is
Solution
2a/Rb
2a/Rb
Q5.∆fH(H2O)= -68 kcal mol-1 and ∆H of neutralization is -13.7 kcal mol-1, then the heat of formation of
Q6.For the gaseous reaction: N2O4 ⟶ 2NO2
Solution
∆H = ∆U + ∆nRT
∆n = nP-nR = 2-1 = 1
∴ ∆H = ∆U + (1)RT
∴ ∆H > ∆U
∆H = ∆U + ∆nRT
∆n = nP-nR = 2-1 = 1
∴ ∆H = ∆U + (1)RT
∴ ∆H > ∆U
Q8.The heat of neutralization of oxalic acid is -25.4 kcal mol-1 using strong base, NaOH. Hence, the enthalpy change of the process is H2C2O4 ⇌ 2H+ C2O42-is
Solution
Oxalic acid has two ionisable H. Hence, expected heat of
neutralization, if it behaves as a strong acid would have been
= -13.7×2 =-27.4 kcal mol-1
But experimental value = -25.4 kcal mol-1
∴ Heat of ionization = 2.0 kcal mol-1
Oxalic acid has two ionisable H. Hence, expected heat of
neutralization, if it behaves as a strong acid would have been
= -13.7×2 =-27.4 kcal mol-1
But experimental value = -25.4 kcal mol-1
∴ Heat of ionization = 2.0 kcal mol-1
Q9.For the process, H2O(s) ⟶ H2O(l) at 273 K
Solution
G(ice) = G(water) = 0
G(ice) = G(water) = 0
Q10. For an endothermic reaction where ∆H represents the enthalpy of the reaction in kJ mol-1, the minimum value for the energy of the activation will be
Solution
Activation energy: Ea is the energy that must be possessed by
the molecules in excess to the average energy at a given
temperature to enter a chemical reaction
Relation between activation energy and enthalpy of a reversible reaction
If the reaction is endothermic in forward direction, then
Ea(backward) = Ea(forward) + ∆H
If the reaction is exothermic in forward direction
Ea(backward) = Ea(forward) + ∆H
For an endothermic reaction, where ∆ H represents the
enthalpy of the reaction in kJ mol-1, the minimum value for
the energy of activation will be slightly more than ∆ H
Activation energy: Ea is the energy that must be possessed by
the molecules in excess to the average energy at a given
temperature to enter a chemical reaction
Relation between activation energy and enthalpy of a reversible reaction
If the reaction is endothermic in forward direction, then
Ea(backward) = Ea(forward) + ∆H
If the reaction is exothermic in forward direction
Ea(backward) = Ea(forward) + ∆H
For an endothermic reaction, where ∆ H represents the
enthalpy of the reaction in kJ mol-1, the minimum value for
the energy of activation will be slightly more than ∆ H